Researchers Decipher the Molecular Mechanism for Endoplasmic Reticulum Shaping Using Deep Learning-Based Predictions
In May 2023, HU Junjie's group at the Institute of Biophysics, Chinese Academy of Sciences, published a research paper titled "Oligomeric scaffolding for curvature generation by ER tubule-forming proteins" in Nature Communications and another one titled "Molecular basis of Climp63-mediated ER lumen spacing" in the Journal of Cell Science. These two publications systematically analyze the molecular mechanism by which endoplasmic reticulum (ER) "tubule-forming proteins" induce membrane curvatures, as well as the structural basis for the regulation of cisternal thickness by ER luminal "bridging proteins."
The ER is an important organelle in eukaryotic cells, responsible for important processes such as protein synthesis, lipid synthesis, and calcium storage. Morphologically, the ER is a continuous membrane system composed by a network of tubules and sheets. The formation of tubular ER relies on the integral membrane proteins of the RTN and REEP families, which induce membrane curvature at the cross-section of tubules and the edges of sheets. The thickness of sheets is primarily determined by Climp63. The shaping of the ER is directly related to its physiological functions. However, these shaping proteins tend to be multimeric and heterogeneous, making it difficult to understand the molecular mechanism.
Using recently developed deep learning-based structure prediction methods, researchers obtained the conformation of the yeast REEP Yop1p and found that elements such as the transmembrane regions are inter-locked in relatively stable positions within the molecule. Subsequently, they deciphered the combination of multiple dimerization interfaces of Yop1p through chemical cross-linking and three-dimensional printing model assembly and discovered that Yop1p multimers have a curved shape that matches ER tubules. In addition, REEP1 is associated with hereditary spastic paraplegia, and its multiple mutations are directly linked to the disruption of REEP1 polymerization, demonstrating the importance of the oligomeric assembly of tubule-forming proteins.
Researchers then applied a similar strategy to predict the structure of Climp63 and found that the luminal domain of Climp63 is primarily helical, with differences in multiple prediction models showing a relatively weak assembly of the helical bundles. They discovered that in one of the conformations, when forming an end-to-end dimer, it has a length consistent with the ER sheet thickness observed in cells. Cross-linking experiments confirmed the existence of this dimerization interface; however, the surface of the intraluminal structural domain of Climp63 is enriched with charged clusters, which may allow for various modes of same-side or opposite-side polymerization. Additionally, the regulation of same-side polymerization of Climp63 was found to be coupled with opposite-side polymerization.
Overall, these works suggest that the shaping of biological membranes typically relies on the regulation of the oligomerization of shaping proteins, which often integrate multiple weak homotypic interactions. This regulatory mode makes the shaping mechanism highly adjustable and flexible. The same theory also applies to the establishment of membrane contact sites in organelle interactions.
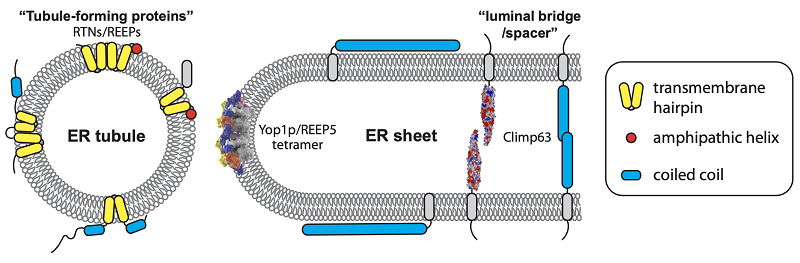
Schematic diagram of the endoplasmic reticulum (ER) shaping molecule and the shaping mechanism
HU Junjie from the Institute of Biophysics, Chinese Academy of Sciences is the corresponding author of the papers, with PhD student XIANG Yun as the first author of the paper on tubule-forming proteins and XU Lu as the first author of the paper on bridging proteins. This series of research is supported by the National Natural Science Foundation of China and the Stable Support Program for Young Talents in Basic Research Field, among others.
Article links:
https://doi.org/10.1242/jcs.260976
https://doi.org/10.1038/s41467-023-38294-y
Contact: HU Junjie
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: huj@ibp.ac.cn
(Reported by Dr. HU Junjie's group)

