HOPE-SIM, a cryo-structured illumination fluorescence microscopy system for accurately targeted cryo-electron tomography
By the Center for Biological Imaging, Core Facilities for Protein Science, Institute of Biophysics, Chinese Academy of Sciences. Cryo-electron tomography (cryo-ET) has become a key technique for investigating in-situ high-resolution structures and their interactions with biological macromolecular complexes. However, cryo-ET data collection requires thinning of cell and tissue specimens to ~200-nm-thick lamellae using a cryo-focused ion beam (cryo-FIB). Further, cryogenic correlative light and electron microscopy (cryo-CLEM) can provide fluorescence-guided localization of target structures during site-specific cryo-FIB milling of frozen-hydrated sections. However, the success rate of cryo-CLEM experiments is constrained by two major factors: the optical resolution of cryo-fluorescence microscopy (cryo-FM) and the precision of the alignment between FM and cryo- electron microscopy (cryo-EM) images.
To resolve the abovementioned technical challenges, the Center for Biological Imaging (CBI), Core Facilities for Protein Science, Institute of Biophysics, Chinese Academy of Sciences (CAS), has been working to develop novel cryo-CLEM techniques. Based on a previously developed high-vacuum optical platform for cryo-CLEM (HOPE) (Journal of Structural Biology, 2017), and incorporating structured illumination microscopy (SIM), we successfully established a cryo-SIM system named HOPE-SIM, which has a lateral optical resolution of better than 200 nm and light microscopy (LM)-FIB three-dimensional (3D) correlation-alignment precision higher than 150 nm. This research achievement was published online in Communications Biology on April 29, 2023.
CLEM uses fluorescent markers to track specific biological macromolecular or subcellular structures, as well as realize 3D FM localization and imaging of the entire cell. Therefore, the FM labeling signals and EM ultrastructures were correlated from the FM and EM images. One of the application directions of cryo-CLEM is the localization of fluorescence-labeled structures in EM images to realize high-resolution EM structural analysis of fluorescence-tracked targets of interest (TOIs). LM is nondestructive to biological specimens; thus, 3D fluorescence localization signals in a specimen can be obtained without destroying it. With the 3D FM images and scanning EM images correlated and matched using a CLEM workflow and correlation-alignment software, fluorescence signal-guided cryo-FIB thinning of the region of interest (ROI) can be realized.
Currently, several types of CLEM have been realized for guiding cryo-FIB thinning. CLEM systems can be classified into two types according to their system structure: separated and integrated LM and EM modalities. The technical team of the CBI has been focusing on methodological research of cryo-CLEM since 2013. For developing CLEM systems with separate LM and EM modalities, a HOPE system was established in 2017 that can be incorporated with an inverted fluorescence microscope (Chinese invention patent number: 201410363314.8; US invention patent number: US9,899,184B2). The HOPE can be adapted to and connected with the cryo-specimen holder (cryo-holder) of a transmission electron microscope (TEM). After completing fluorescence localization of a cryo-specimen, the cryo-specimen, along with the cryo-holder, is transferred to an TEM for high-resolution data collection. In addition, wide field optical images and TEM images can be matched using LM-TEM correlation-localization software. This effectively avoids repeated manipulation of the cryo-grid during CLEM imaging process, ensures the integrity and consistency of the cryo-specimen, and effectively improves the correlation success rate and experiment efficiency. This achievement was published in the Journal of Structural Biology in 2017.
However, owing to the light diffraction limit and the limited space in cryo-FM, the wide-field microscopy-based HOPE system can only use a cryo-FM system with a long working distance and a low numerical aperture (NA). Consequently, it only has a lateral resolution of ~400-500 nm, rendering it unsuitable for capturing several-hundred-nanometer-scale target structures in several-micron-thick cells.
Super-resolution structured illumination fluorescence microscopy (SIM) can increase the resolution of wide-field fluorescence microscopy by one-fold and offers the following technical advantages: no need for special fluorescent probes, rapid imaging, and low illumination density. Therefore, among all high-resolution microscopy techniques, SIM is the most suitable for high-resolution imaging of cryo-specimens under cryogenic conditions. Accordingly, the technical team of the CBI selected SIM as a way to improve the resolution of cryo-FM. We developed a large-chamber compatible with inverted fluorescence microscopes; integrated with a 0.9 NA long-working-distance optical objective lens, an anti-contamination system (ACS), and externally connected to a vacuum transfer turbo pump system (TPS) and a cryo-holder adapter. In addition, 3D SIM imaging of cryo-specimens under vacuum conditions was realized, resulting in improved resolution of cryo-LM and protection of the cryo-specimen during its transfer for CLEM. Figure 1 presents the design and hardware architecture of the HOPE-SIM system.
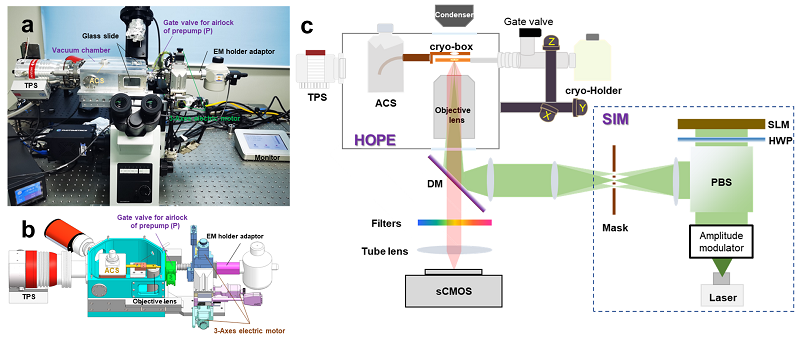
Figure 1 Cryo-SIM system (HOPE-SIM)
a. Hardware architecture of HOPE-SIM; b. Schematic of design principle for HOPE-SIM; c. Schematic of principle for HOPE-SIM
Using the HOPE-SIM cryo-CLEM system and self-designed 3D correlation software (3D-View), the technical team of the CBI successfully prepared cryo-lamellae targeting herpesvirus assembly compartment of infected BHK-21 cells (Figure 2) and centrosomes of HeLa cells(Figure 3) , and analyzed their in-situ structures by cryo-ET data collection and analysis. From the experimental results, the HOPE-SIM cryo-CLEM workflow realized a 3D alignment precision of better than 150 nm and provides an efficient, accurately targeted cryo-FIB milling technical solution for in-situ capturing of large-size, high-intracellular-abundance TOIs.
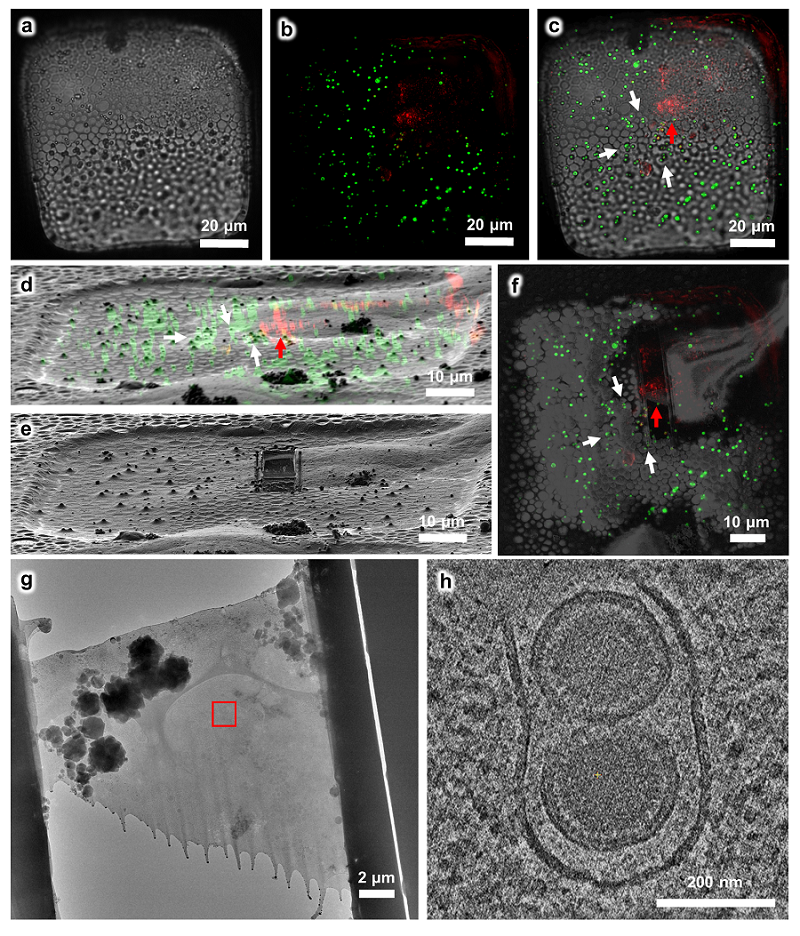
Figure 2 Using the HOPE-SIM-based cryo-CLEM workflow to capture the MHV-68 viral particles in host cells
a. Bright-field image of the target square. b. The z projection of HOPE-SIM fluorescent image. Green, fluorescent microspheres. Red, MHV-68 virus. c. The fluorescent image in (b) is merged with the bright-field image in (a) to show the location of target signal. d. 3D correlation between the cryo-SIM and cryo-FIB images. e. Cryo-FIB image of the target square after fabrication. f. Cryo-SEM image of the target square after fabrication, which is merged with the z projection of cryo-SIM image in (b). g. Cryo-EM micrograph (×3,600) of the cryo-lamella in (f). The target region marked by the red rectangle is subjected for cryo-ET reconstruction in (h) with a magnification of ×64,000, where one slice to the tomogram shows different types of viral particles that are under tegumentation. See Supplementary Data 6 for raw cryo-FIB images of (d) and (e), raw cryo-SEM image of (f), and raw cryo-EM image of (g).
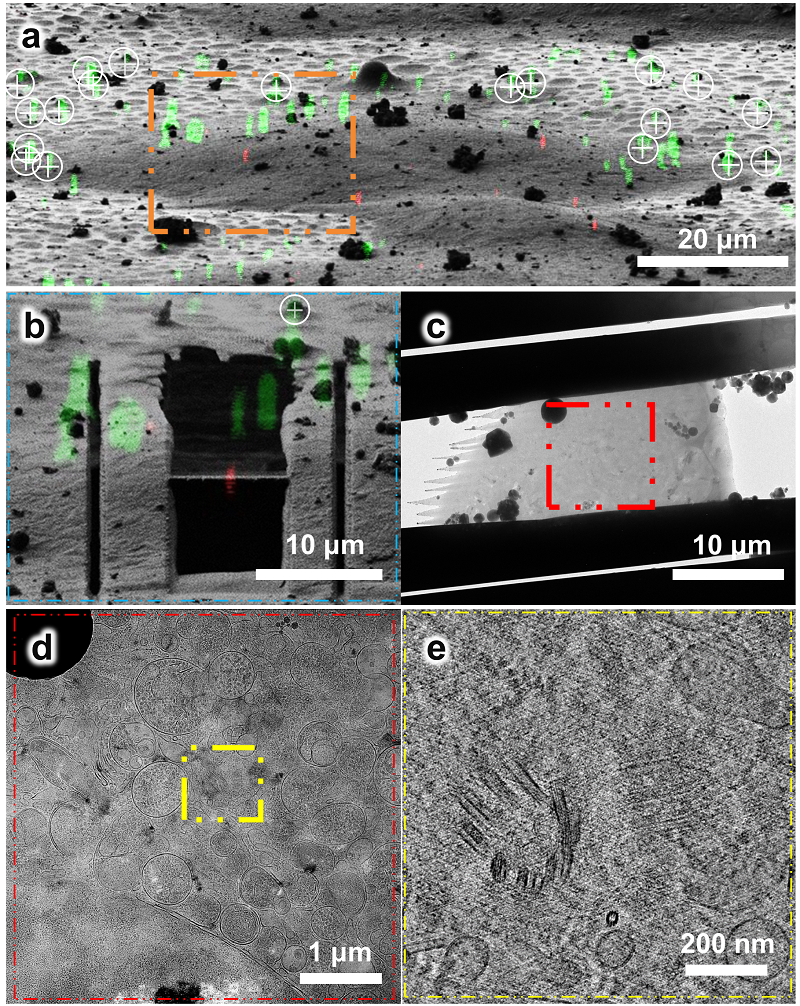
Figure 3 Using the HOPE-SIM-based cryo-CLEM workflow to capture the centrosomes in HeLa cells.
a. 3D correlation between HOPE-SIM and cryo-FIB images to localize the positions of the selected centrosomes.; b. Cryo-FIB milling of red fluorescence-marked ROI; c. Cryo-EM micrograph of the C3 cryo-lamella with the magnification of ×4300; d. Cryo-EM micrograph (×8700) of the C3 cryo-lamella at the region of the red square in (c). The target region marked by the white square is subjected for cryo-ET data collection and reconstruction; e. One slice of the tomogram (×53,000) of the target region, showing the target centrioles with one in the top view and another in the side view.
Professor-Level Senior Engineer Ji Gang and Professor Sun Fei at the CAS Institute of Biophysics are the corresponding authors of this article. Senior Engineer Li Shuoguo at the CBI is the first author of this article. Engineer Jia Xing at the CBI participated in the cryo-FM specimen preparation. Engineer Niu Tongxin participated in the data processing. Engineer Assistants Zhang Xiaoyun and Qi Chen participated in the data processing of cryo-LM imaging and cryo-EM imaging, respectively. Professor Xu Wei and Deng Hongyu at the Institute of Biophysics provided invaluable guidance on this study. Special appreciation is extended to Professor Ji Wei and Li Dong at the Institute of Biophysics for their guidance and help with optical imaging techniques. Further, this study was supported by grants from the Ministry of Science and Technology of China, the Strategic Priority Research Program of Chinese Academy of Sciences and the National Natural Science Foundation of China. In addition, this work was supported by the Technological Innovation Program of the Chinese Academy of Sciences, the CAS Key Technical Support Personnel Project. The specimen preparation, data collection, and analysis were supported by the technical engineer team of the CBI.
URL of article: https://www.nature.com/articles/s42003-023-04850-x
It is worth mentioning that regarding the development of integrated CLEM systems, the CLIEM system established by the team of Prof. Xu Tao and Prof. Ji Wei at the Institute of Biophysics and the three-beam (electron, light, and ion beams) trifocal microscopy (ELI-TriScope) system developed by the technical team of the CBI were both reported in the Nature Methods in January 2023. An optical imaging system is integrated into the vacuum chamber of a dual-beam scanning electron microscope(SEM), thereby avoiding the specimen transfer and effectively improving the precision and success rate of cryo-CLEM. A cryo-specimen holder-based transfer system (cryo-transfer system) is integrated into the ELI-TriScope system developed by the technical team of the CBI, and an inverted FM system, named cryogenic SimulTAneous monitoR (cryo-STAR) system, is embedded beneath the cryo-specimen, which accurately focuses the electron, light, and ion beams at the same point and monitors the fluorescence signals of the target molecules in real-time during cryo-FIB milling. This significantly improves the precision of capturing specific TOIs during cryo-FIB milling and decreases the time cost of preparing a frozen-hydrated section from 1.5 -2 h.
The HOPE-SIM system developed by the technical team of the CBI is capable of 3D high-resolution cryo-FM imaging and can be directly connected to the ELI-TriScope system using a cryo-holder. In addition, the system is capable of 3D high-resolution FM imaging and real-time FM monitoring of the entire FIB milling process. As a result, the efficiency, accuracy, success rate, and throughput of cryo-FIB milling were effectively improved, and a successful solution for in-situ structural analysis was provided, demonstrating a huge potential for corresponding application in in-situ structural biology.
URLs of articles:
ELI TriScope: https://www.nature.com/articles/s41592-022-01748-0
CLIEM: https://www.nature.com/articles/s41592-022-01749-z
URLs of press releases:
http://www.ibp.cas.cn/kyjz/zxdt/202301/t20230113_6599317.html
http://www.ibp.cas.cn/kyjz/zxdt/202301/t20230113_6599093.html
Contact: LI Shuoguo
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: lishuoguo@ibp.ac.cn
(Reported by the Center for Biological Imaging)

