Prof. CHEN Chang Cooperates with Profs. ZHOU Xiao-Long to Reveal that Mitochondrial Translational Defects Extend Lifespan in Caenorhabditis elegans by Activating UPRmt
Eukaryotes have at least two protein synthesis systems and require two sets of aminoacyl-tRNA synthetases (aaRSs). Typically, these two sets, one in the cytoplasm and the other in the mitochondria, are encoded by nuclear genes. It is relatively rare for a single gene to code for cytoplasmic and mitochondrial aaRSs for a given amino acid; and the specific mechanism still requires further investigation. On May 7, 2023, the international academic journal Redox Biology published the latest findings from a collaborative research. The research involved Dr. ZHOU Xiao-Long's group from the Chinese Academy of Sciences Center for Excellence in Molecular Cell Science (Institute of Biochemistry and Cell Biology) and Dr. CHEN Chang's group from the Institute of Biophysics, Chinese Academy of Sciences. The paper is titled "Mitochondrial translational defects extend lifespan in Caenorhabditis elegans by activating UPRmt." The study presents the first case of a single gene encoding cytoplasmic and mitochondrial threonyl-tRNA synthetases (ThrRSs) by mRNA translation re-initiation. The study revealed that mitochondrial translation defects extend the lifespan of C. elegans by activating the mitochondrial unfolded protein response (UPRmt). Moreover, it revealed that the activation of UPRmt by the mitochondria translational defect associated with aaRS deficiency is universal and conserved among species.
The researchers first analyzed the gene and protein isoforms of ThrRS in C. elegans and found only one potential ThrRS gene, tars-1, which encodes two ThrRSs of varying lengths. Through Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and 5'-Rapid Amplification of cDNA Ends (5'-RACE), it was confirmed that there was only one tars-1 mRNA in C. elegans, which could produce two ThrRS isoforms through translation re-initiation. Fluorescence localization experiments in mammalian cells confirmed that the longer and shorter isoforms of ThrRS (CeThrRS-1 and CeThrRS-2) were localized to the mitochondria and cytoplasm, respectively. By constructing two yeast genetic mutants, they demonstrated in vivo that tars-1 produces CeThrRS-1 and CeThrRS-2 through translation re-initiation, each functioning in protein synthesis in the mitochondria and cytoplasm, respectively. They systematically studied cytoplasmic and mitochondrial tRNA aminoacylation and quality control mechanism mediated by CeThrRS-2. To investigate the physiological function of mitochondrial tars-1, CRISPR-Cas9 was used to obtain a C. elegans strain with mitochondrial tars-1 knocked down. The knockdown of mitochondrial tars-1 in C. elegans led to developmental delays, decreased mobility, reduced egg-laying, and an extended lifespan. Further studies revealed impaired mitochondrial function, including decreased oxygen consumption rate, diminished Complex I activity, mitochondrial redox stress, activation of UPRmt, and extended lifespan of C. elegans depending on UPRmt activation. Further knockdown of various mitochondrial aaRSs in C. elegans and mammalian cells showed that all could activate UPRmt, indicating the generality and conservation of UPRmt activation due to mitochondrial aaRS defect-related mitochondrial translation deficiency.
Mitochondria, as semi-autonomous organelles, have a genome (mitochondrial DNA or mtDNA) responsible for encoding a small number of crucial protein subunits. These proteins are critical to the mitochondrial oxidative phosphorylation complex, playing a significant role in the biogenesis, structure, and functional regulation of mitochondria. Given the central role of mitochondria in cellular energy supply, metabolic regulation, and cell fate decisions, disorders in mitochondrial protein synthesis in humans frequently lead to diseases that affect multiple tissues and organs. These include neurodegenerative diseases, heart diseases, muscle weakness, epilepsy, deafness, and reproductive defects, collectively called mitochondrial encephalomyopathy. However, our understanding of the biological functions of mitochondrial protein synthesis in other eukaryotic model organisms is limited. This study provides systematic insights into the molecular mechanisms of two distinctly localized ThrRSs encoded by a single ThrRS gene from the levels of genes, transcripts, protein isoforms, biochemical mechanisms, cellular localization, organelle function, and the whole organism. It establishes and elucidates the mechanism of protein synthesis speed and quality control mediated by nematode ThrRS for the first time, reveals that mitochondria translational defects can extend the lifespan of nematodes by activating mitochondrial UPR, and underscores the universal principle that mitochondrial aaRS defects activate UPRmt. This research provides a new foundation to deepen our understanding of the key role of mitochondrial protein synthesis in eukaryotic aging and related diseases.
Profs. ZHOU Xiao-Long and CHEN Chang are the corresponding authors of this paper. Ph.D. GUO Miaomiao; Senior Engineer QIAO Xinhua; Postdoctoral WANG Yuanyuan from the Institute of Biophysics, CAS; and Ph.D. LI Zi-Han from CAS Center for Excellence in Molecular Cell Science, are the co-first authors. The group would like to specially acknowledge Prof. WANG Enduo from the CAS Center for Excellence in Molecular Cell Science and Prof. TIAN Ye from the Institute of Genetics and Developmental Biology, CAS, for their invaluable supports. The project has received funding from the National Key R&D Program, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, Committee of Science and Technology in Shanghai, CAS Project for Young Scientists in Basic Research.
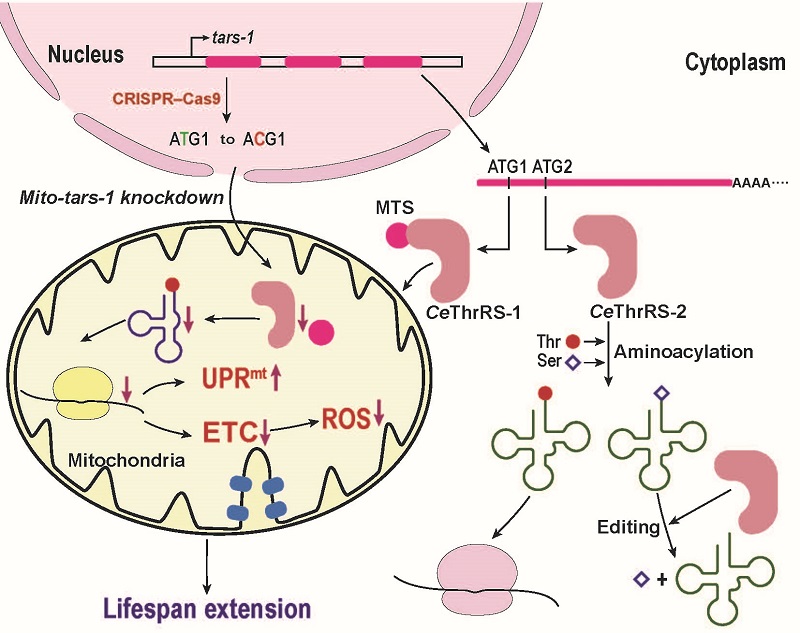
Figure: Mitochondrial translational defects extend lifespan in C. elegans by activating UPRmt
Link to the article: https://doi.org/10.1016/j.redox.2023.102722
Contact: CHEN Chang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: changchen@ibp.ac.cn
(Reported by Dr. CHEN Chang's group)

