Structure of moss photosystem I reveals evolutionary adaptation to changing light conditions during aquatic-terrestrial transition
Land plants evolved from green algae during the transition from aquatic to terrestrial environments. Bryophytes, including liverworts, mosses and hornworts, distinguished themselves in this evolutionary event as the first types of terrestrial plants. The moss Physcomitrium patens (P. patens) has been used extensively as a model plant in a wide range of research fields.
Photosystem I (PSI) and Photosystem II (PSII) are important photosynthetic apparatuses responsible for capturing, transferring and converting solar energy in the process of photosynthetic light reactions. Both photosystems consist of a core complex and light-harvesting complexes (LHCs). Compared with green algae and flowering plants, P. patens has a unique and diverse composition of its light-harvesting antennae, which may be beneficial for its adaptation to environmental changes. Recent studies have shown that P. patens contains at least three different types of PSI with variable antenna sizes, and the largest PSI (PpPSI-L) shows a unique composition not found in either flowering plants or green algae. The structural analysis of PpPSI-L will provide important basis for revealing the mechanism of early plants adaptation to terrestrial environments.
In the study published in Nature Plants, Researchers from the Institute of Biophysics, Chinese Academy of Sciences and the Capital Normal University reported the high-resolution cryo-electron microscopic structure of PpPSI-L. They identified 26 protein subunits in this supramolecular complex, namely 14 PSI core subunits, one P. patens-specific LHC protein Lhcb9, one phosphorylated LHCII trimer, and eight LHCI monomers arranged as two non-parallel belts (each belt consists of four LHCI proteins) (Fig.1a). Based on the positions and orientations of pigment molecules revealed by the structure, researchers constructed the potential energy transfer pathways within the complex and calculated the energy transfer rates between different subunits (Fig.1b).
The PpPSI-L structure confirms and explains the important role of Lhcb9 and phosphorylated LHCII in stabilizing the complexes. In addition, researchers found that the inner and outer LHCI belts are formed by the same gene products which are arranged in the same order. Moreover, the PpPSI-L complex was found to be internally flexible, with LHCII and the outer belt bending in opposite directions relative to the PpPSI-S moiety (the PSI core plus the inner LHCI belt). These structural features suggest that different types of P. patens PSI may share identical modules, enabling fast switch between types, which may contribute to the dynamic adjustment of the light-harvesting capability of PSI under different light conditions.
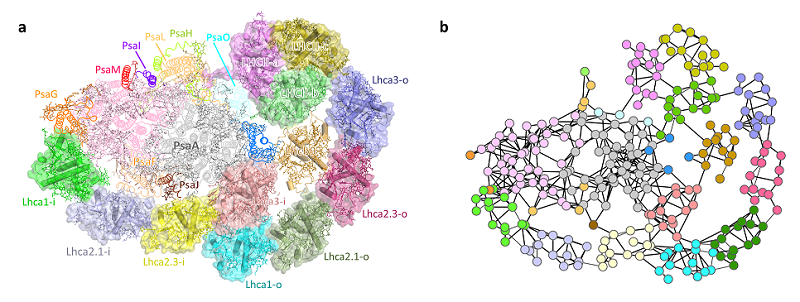
Fig 1. Overall structure of PpPSI-L (a) and potential energy transfer pathways (b)
Article link: https://www.nature.com/articles/s41477-023-01463-4
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: meili@ibp.ac.cn
(Report by Prof. LI Mei's group)

