Researchers reveal the role of protein oxidative folding in stem cell aging
It has been widely acknowledged that mitochondrion is the main source of reactive oxygen species (ROS) for a long time. The role of the endoplasmic reticulum (ER)-generated ROS has been less extensively studied. It has been proposed that oxidative protein folding contributes approximately 25% of cellular ROS during protein synthesis. Thus, the ER-derived ROS cannot be ignored. Yet the role of the ER-derived ROS in the regulation of stem cell senescence remains unknown.
On 3 Aug, 2023, Prof. WANG Lei/WANG Chih-chen's group at the Institute of Biophysics, Chinese Academy of Science (CAS) and Prof. LIU Guang-Hui's group at the Institute of Zoology, CAS published a research article in EMBO Reports entitled Reducing oxidative protein folding alleviates senescence by minimizing ER-to-nucleus H2O2 release (cover story). This study established the relationship between oxidative protein folding and stem cell aging for the first time. It was observed that H2O2 generated by oxidative protein folding in the ER can be released to the nucleus, therefore induced the expression of SERPINE1, a key factor promoting cell senescence.
The researchers found that protein disulfide isomerase (PDI), a key oxidoreductase that catalyzes oxidative protein folding, accumulated in aged human cells and the liver of mice, and depletion of PDI alleviated cell senescence. Furtherly, by using an ultra-sensitive, genetically encoded fluorescence probe, researchers showed that PDI deficiency slowed the rate of oxidative protein folding and reduced the level of its byproduct H2O2 both in the ER and the nucleus. Through omics analysis, researchers found that PDI deficiency causes the downregulation of SERPINE1, an aging-related molecule regulated by H2O2, thereby alleviating the senescence of stem cells. Finally, the researchers also showed that knockdown of PDI in various human cell models can delay senescence, indicating that PDI may be a potential molecular target for alleviating aging.
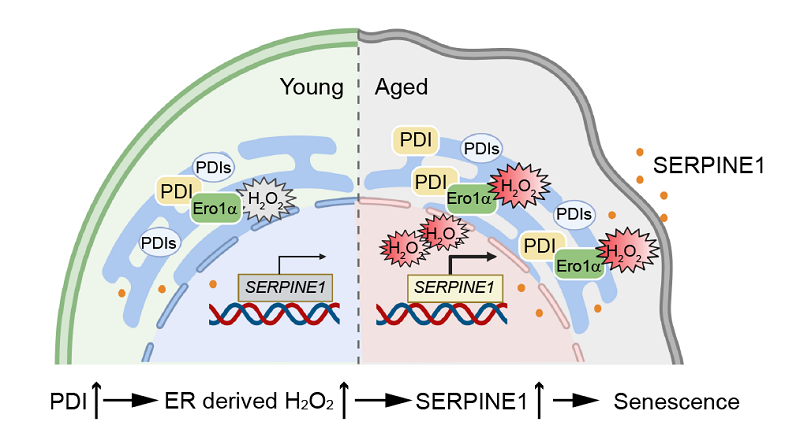
Figure 1: H2O2 generated by oxidative protein folding in the ER can be released to the nucleus and promotes cell senescence.
Previous studies have revealed that reducing the transcription and protein synthesis alleviate senescence. This study provides new ideas and molecular targets for understanding aging from the perspective of protein folding, implying that in order to achieve "sustainable development of individuals" (younger), cells should also improve "energy efficiency and emission reduction" (reducing oxidative protein folding).

Figure 2: Cover story: reducing oxidative protein folding alleviates senescence. The aging of a cell is similar to the development of a city. The bottom part of the illustration shows that during industrialization, the workers (PDI and Ero1 enzymes) in the factory (the endoplasmic reticulum) produce not only steel and concrete (disulfide-containing proteins) for city construction, but also produce a lot of pollutants (H2O2). The top part of the illustration shows a healthy and very green landscape with a small "house factory", implying that depletion of PDI results in slower but safer oxidative protein folding to alleviate cell senescence.
Profs. WANG Lei from the Institute of Biophysics, CAS and LIU Guang-Hui from the Institute of Zoology, CAS are the co-corresponding authors of the paper. Ph.D. CHENG Fang from the Institute of Biophysics, CAS, and Ph.D. JI Qianzhao from the Institute of Zoology, CAS, are the co-first authors. The study is supported by the National Key R&D Program of China; the National Natural Science Foundation of China; the Strategic Priority Research Program of CAS and the Youth Innovation Promotion Association, CAS.
Article link: https://www.embopress.org/doi/full/10.15252/embr.202256439
Contact: WANG Lei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wanglei@ibp.ac.cn
(Reported by Prof. WANG Lei/WANG Chih-chen's group)

