Scientists Unveil the Mechanism of Nucleosome Assembly by Chromatin Assembly Factor-1
Chromatin inheritance during cell division involves replication of DNA and assembly of nucleosomes onto replicated DNA, because passage of the DNA replication fork disrupts nucleosomes on the DNA. Half of the histones for the replication-coupled nucleosome assembly comes from the disrupted parental nucleosomes, and the other half is newly synthesized. Chromatin assembly factor-1 (CAF-1) is an evolutionarily conserved heterotrimeric protein complex that is responsible for the deposition of newly synthesized histones H3 and H4 onto DNA. However, the lack of structural information about CAF-1 has hindered the understanding of the molecular mechanism underlying de novo nucleosome assembly.
On August 25, 2023, a groundbreaking study conducted by the research team led by Prof. XU Ruiming at the Institute of Biophysics (IBP), Chinese Academy of Sciences, in collaboration with Prof. LI Guohong, Prof. ZHU Bing and Prof. LIU Chaopei, all at IBP, reported high-resolution structures of CAF-1 and CAF-1 bound to histones H3-H4 in a research article, entitled "Structural insights into histone binding and nucleosome assembly by chromatin assembly factor-1", in the Science journal.
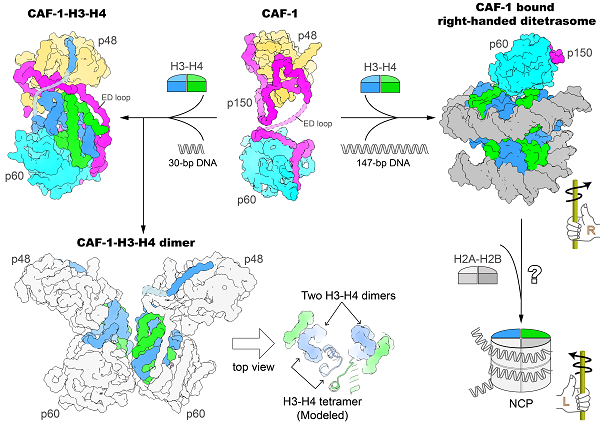
Figure: Structures of CAF-1 and CAF-1 bound with histone H3-H4
The researchers determined the crystal structure of the core domain of human CAF-1 in the absence of histones and the cryo-EM structure of CAF-1 in complex with histones H3 and H4. Their results show that a CAF-1 complex binds to an H3-H4 heterodimer mainly through the p60 subunit and the ED-loop of the p150 subunit. The C-terminal segment of the ED-loop is key to the biological function of CAF-1, judged through corroborating the structural information with in vitro histone binding and plasmid supercoiling assays, as well as in vivo nascent nucleosome assembly mapping and transcriptome analyses.
They also find that a 30-bp DNA oligomer triggered the dimerization of the CAF-1-H3-H4 complex and they solved the cryo-EM structure of a 2:2 CAF-1-H3-H4 complex. This structure shows that two adjacent H3-H4 heterodimers are poised for the formation of an H3-H4 tetramer via dimerization of two H3s, but the positioning of the two heterodimers are not yet in the exact geometric arrangement of an H3-H4 tetramer, suggesting that a remodeling of histone H3-H4 binding by CAF-1, perhaps with the binding of a longer DNA fragment, is needed to assemble the H3-H4 tetramer, which is an essential unit of the nucleosome.
Surprisingly, using a 147-bp DNA fragment the researchers observed a CAF-1-bound right-handed di-tetrasome structure. This discovery was further confirmed by the analysis using the single-molecule Freely Orbiting Magnetic Tweezer (FOMT) method at a physiological salt concentration. This discovery indicates the possible involvement of a right-handed nucleosome precursor in replication-coupled nucleosome assembly, and points a new direction of mechanistic investigation of the nucleosome assembly process.
Article link: https://www.science.org/doi/epdf/10.1126/science.add8673
Contact: LIU Chaopei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: liuchaopei@ibp.ac.cn
(Reported by Prof. XU Ruiming's group)

