The group of Prof. ZHU Ping and collaborators revealed a unique, hitherto-unknown bacterial transcriptional promoter recognition mode by distinct σI factors
On Oct 13, 2023, Nature Communications published a research paper by researchers lead by Prof. ZHU Ping at the Institute of Biophysics, Chinese Academy of Sciences and Prof. FENG Yingang at the Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences. The paper by Li et al. entitled "Structure of the transcription open complex of distinct σI factors" reveals a unique, hitherto-unknown bacterial transcriptional promoter recognition mode by distinct σI factors in C.thermocellum, both with respect to domain organization and binding mode to promoter DNA.
Lignocellulose can be efficiently degraded by a class of anaerobic clostridia represented by C.thermocellum through a secreted multienzyme complex, termed cellulosome, which is of great value in the development of bioenergy. Bacterial σ factors are critical components of RNA polymerase (RNAP) holoenzymes for initiation of transcription by specifically recognizing DNA promoter regions. A single bacterium often contains multiple σ factors. However, the σI (SigI) in C. thermocellum exhibits lower homology to most σ factors and stands as a unique member within the bacterial σ factor family σ70 (Figure 1A).
To reveal the molecular mechanism how the SigI factors in C.thermocellum recognize promoters, researchers prepared a ternary complex formed by SigI, RNAP, and promoter DNA, and determined the cryo-EM structures of RNAP-σ-promoter complexes (transcription-ready open complexes, RPo complexes) of two C.thermocellum σI complexes, i.e., RPo-SigI1 and RPo-SigI6 complexes, in the active state at 3.0 Å and 3.3 Å resolution, respectively. Comparing the structures of these two RPo-SigI complexes with those of other transcriptional complexes, the researchers found that although the SigI-mediated transcriptional open complex presents an overall conserved architecture, SigI exhibits a distinctive promoter recognition mechanism among other σ factors in σ70 family (Figure 1B). Firstly, the C terminal domain of SigI (SigIC) presents a unique -35 element recognition mode. SigIC binds the DNA major groove of promoter -35 element through its helix-turn-helix (HTH) motif, which is rotated about 180° compared to the HTH binding in the major groove of σ4 domain of other σ factors in σ70 family. In addition to the binding with the major groove, SigIC also inserts a conserved histidine into the DNA minor groove of -35 element, which is the conserved A-tract region of the promoter, representing a unique promoter recognition mode of SigI factors. Secondly, the recognition mode to the promoter -10 element by the N terminal domain of SigI (SigIN) also presents novel features: the SigIN domain has a conserved interaction mode with CGWA of -10 element while all transcription-bubble bases in -10 element interacting with SigIN are flipped out, which forms extensive protein-nucleic acid interactions with SigIN. These interactions may modulate the promoter activity and determine the differences in the transcription strength of SigI-dependent promoters of cellulosomal genes.
Putting together, the above results indicate that the transcriptional regulatory factor σI in C.thermocellum possesses a unique promoter recognition mechanism. This finding holds significant implications for understanding how C.thermocellum regulates the expression of cellulase genes and for the modification and application of its cellulases. Additionally, the distinctiveness of σI factor in its promoter recognition mechanism enriches our understanding of the diversity of microbial transcriptional regulation.
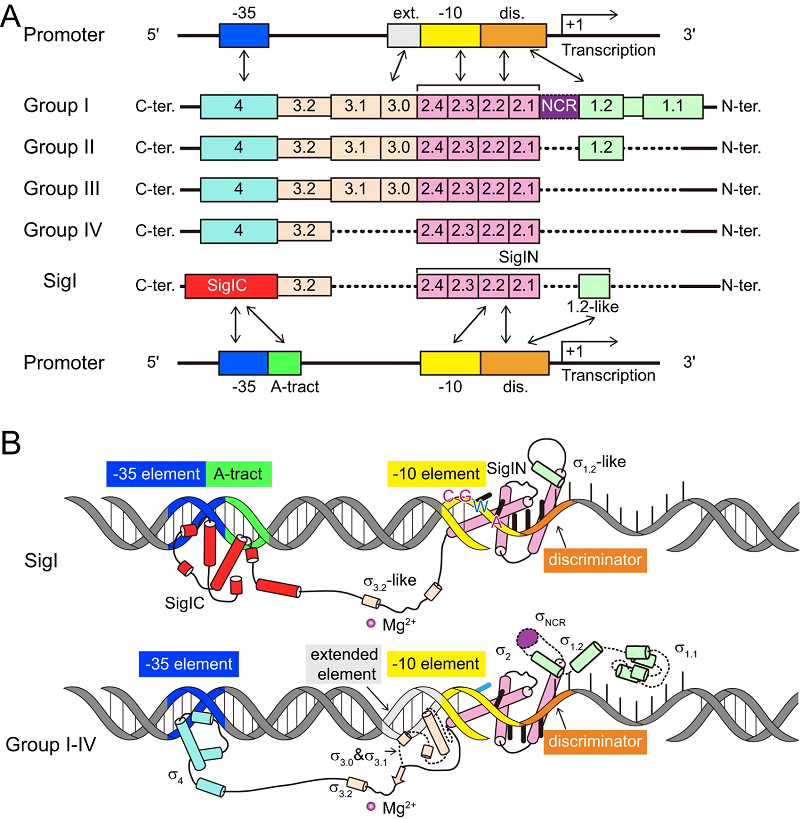
Figure 1: Schematic diagrams of the promoter recognition by σI and by the four groups of σ70 family σ factors.
ZHANG Haonan, a Ph.D. student of the ZHU Ping's group, and LI Jie, a Ph.D. student of the FENG Yingang's group are the co-first authors of the Nature Communications paper. Prof. ZHU Ping and Prof. FENG Yingang are the corresponding authors of the paper. This research work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of Chinese Ministry of Science and Technology, and the Strategic Pioneering Science and Technology Special Project of the Chinese Academy of Sciences.
Original article link:
https://www.nature.com/articles/s41467-023-41796-4
Contact: ZHU Ping
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhup@ibp.ac.cn
(Reported by Prof. ZHU Ping's group)

