Researchers identify the initiation mechanism for ER-phagy induced by misfolded proteins
The endoplasmic reticulum (ER) is the largest organelle in eukaryotic cells, serving as a major site of protein folding and maturation. ER-phagy, lysosomal degradation of obsolete ER portions, is crucial for protein quality control and turnover of the organelle to maintain its functionality. The selective recognition of ER by autophagic system is directed by ER-phagy receptor proteins. However, most ER-phagy receptors lacking a luminal domain cannot directly sense the signals originating from the ER lumen to initiate ER-phagy, but play a downstream execution role. The initiation mechanism for ER-phagy is still a key question awaiting an answer.
On Nov2, 2023, Prof. WANG Chih-chen /WANG Lei's group from the Institute of Biophysics, Chinese Academy of Sciences (CAS), published a research article entitled "V-ATPase recruitment to ER exit sites switches COPII-mediated transport to lysosomal degradation" in Developmental Cell. They report that accumulation of misfolded Z-AAT at the ER exit sites (ERESs) inhibits ER-Golgi transport, and activates the V-ATPase-ATG16L1-LC3C axis to recruit FAM134B-II to initiate ER-phagy.
Z-AAT, a disease-causing mutant of α1-antitrypsin, induces noncanonical ER-phagy at ERESs. Accumulation of misfolded Z-AAT at the ERESs impairs COPII-mediated ER-to-Golgi transport, and retains V0 subunits that further assemble V-ATPase at the arrested ERESs. V-ATPase subsequently recruits ATG16L1 onto ERESs to mediate in situ lipidation of LC3C. FAM134B-II is then recruited by LC3C via its LIR motif and elicits ER-phagy leading to efficient lysosomal degradation of Z-AAT. Activation of this ER-phagy mediated by V-ATPase-ATG16L1-LC3C axis (EVAC) is also triggered by blocking ER export.
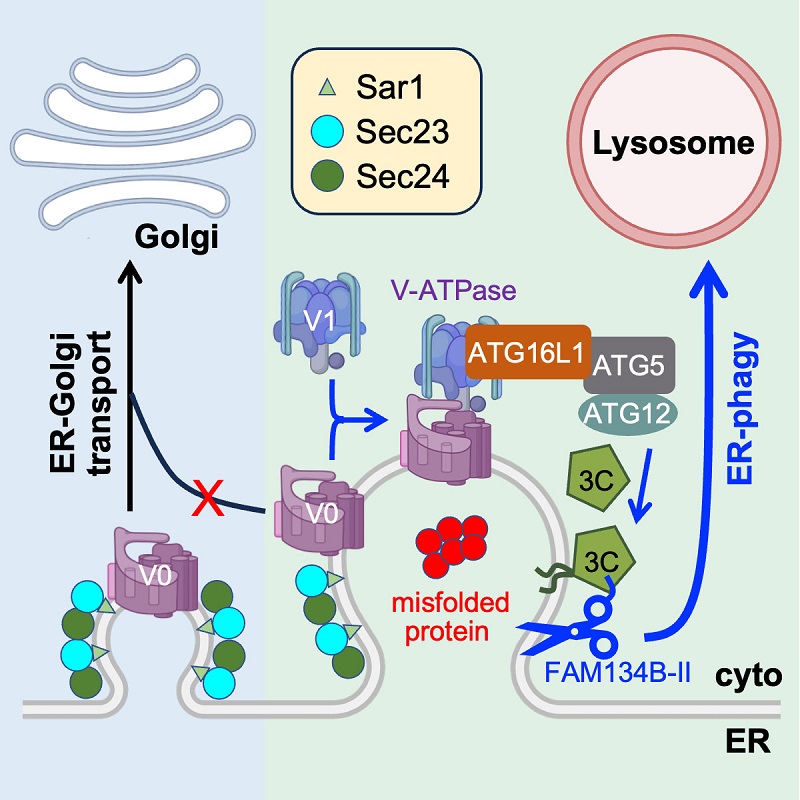
Figure. The initiation mechanism for ER-phagy induced by misfolded proteins
This study shows that when the COPII-mediated ER-Golgi transport is blocked, the arrested ERESs can be switched to ER-phagy sites for subsequent delivery to lysosomes for degradation. This work uncovers a functional switch of ERESs to ER-phagy as the last line of defense in ER quality control, and provides insights into the crosstalk between the secretory pathway and autophagy.
Dr. WANG Xi is the corresponding author of the paper, and Dr. SUN Yiwei is the first author. This work was supported by the National Natural Science Foundation of China, the National Key R&D Program of China, the Strategic Priority Research Program of CAS, and the Project for Young Scientists in Basic Research of CAS.
Article link: https://doi.org/10.1016/j.devcel.2023.10.007
Contact: WANG Xi
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wangxi@ibp.ac.cn
(Reported by Prof. WANG Lei/WANG Chih-chen’s group)

