SUN Fei's group reveals the in-situ structure of mouse sperm axoneme double microtubules
Axonemes are the motility basis of motile cilia. They exist widely in unicellular and multicellular organisms and are mainly responsible for cell movement, intercellular communication, sensory reception, embryonic development and other important life activities. The axoneme is a typical "9+2" structure, consisting mainly of the Central pair complex (CPC) and nine surrounding Microtubule doublets (DMT). There are radial spokes (RS) in the periphery of DMT connecting it with CPC, as well as Outer dynein arm (ODA) and Inner dynein arm (IDA) which provide power for axoneme movement. The structural and functional abnormalities of each component of the axoneme may lead to Primary ciliary dyskinesia (PCD) and asthenospermia. Sperm need to overcome the resistance of mucus and mechanical external force in the process of sperm-egg recognition. Therefore, the integrity and stability of the axoneme are very important for the formation of the fertilized egg, and the DMT distributed in the outer part of the axoneme also plays an important role. Recently, the single-particle structure of sperm DMT of different species has been reported successively, but the lack of high-resolution in-situ structure reveals the role of Microtubule inner protein (MIP) of sperm DMT in maintaining its stability and coordination during sperm swimming.
A research paper titled "In-cell structural insight into the stability of sperm microtubule doublet" was published online on Cell Discovery by a team led by SUN Fei from the Institute of Biophysics, Chinese Academy of Sciences. In this paper, they report the in situ structure of mouse sperm axoneme microtubule doublet with the resolution of 4.5-7.5 angstroms and identify 36 kinds of MIPs. Combined with the deformed axoneme DMT in situ structure, the mechanical role of different MIPs in the deformation of DMT was revealed. This work provides a structural basis for sperm motility and new insights into the treatment of male infertility related diseases.
Sperm is a kind of cell with special function and shape. It carries the genetic material and proteins of the male partner, finds and fuses with the female oocyte at fertilization. Mature mammalian sperm consists of two main parts: a head with a nucleus and an acrosome, and a long tail for movement. The tail of the sperm is also called the motile cilia or flagella, and its core component is the axoneme, which is a very large molecular machine based on microtubules. There are hundreds of different proteins modified on microtubule-based structures to form various functional components. The motor protein motor is responsible for driving sperm movement and fine tuning movement, while the MIP provides the main stabilizing force for the DMT structure. Studies of cilia in different species have shown that having MIP in DMT is a common feature of different life forms, but the form of these MIP varies between species. Recent studies using Cryo-EM and single particle analysis (SPA) techniques have provided detailed structures of purified DMT from different sources. These studies revealed a wide variety of MIP and other structural features. Unlike tracheal cilia, mammalian sperm rely on the powerful pulsation of the flagella to overcome obstacles in the female reproductive tract. Thus, to accomplish this extreme task, sperm employ several special features, such as long axons, spiral-shaped mitochondria in the energy-producing tail, and a complete set of MIP within the mitochondria to provide stability for the axoneme DMT.
In order to explore the details of in situ high resolution assembly of mammalian sperm axoneme, the in situ structure of mouse sperm axoneme DMT was analyzed using a combination of cryo-focused ion beam (Cryo-FIB) milling, Cryo-ET and STA, artificial intelligence (AI) assisted modeling and mass spectrometry (MS). A total resolution of 4.5 ~ 6.5 angstrom was obtained for 16 nm repeats and 6.5 ~ 7.5 angstrom for 48 nm repeats, and models of 16 nm and 48 nm repeats were established. 36 different MIPs were identified in intact mouse sperm DMT that play a role in strengthening the DMT lumen, providing insight into how mammalian MIP weaves a robust network of interactions to give DMT structural stability.
This study focuses on the Tektin protein bundle as the center, and the other MIPs stabilize the Tektin bundle in the A tubule of DMT through complex interactions to enhance its stability. Tektin1-Tektin4 was identified in the DMT of the trachea cilia, extending lengthwise along the DMT. Tektin5 specifically expressed in testis and sperm further filled the density of A tubule compared to bovine trachea DMT, Tektin5 existed in 7 different structural morphologies in sperm, and further consolidated Tektin bundles at the weak "junction site" of Tektin1-Tektin4. NME7, CFAP141, CFAP161, EFHC1, EFHC2, FAM166A, MNS1 and other MIPs reinforce Tektin bundles to be stabilized on Tubulin from all directions, which may be related to the complex swimming process that sperm need to complete. The ODA, IDA, RS, and B tubule are all attached to the A tubule and therefore provide important support functions for the coordination and stability of the components in the motion of the axoneme. Compared with the rich composition of MIP in A tubule, only 11 kinds of MIP, such as CCDC105, TEX43, CFAP77 and SPACA9, were identified in B tubule, and a specific period of SPACA9 manifested as "5-3-3-4-4-4" was also proposed in this study.
In addition, another in-situ structure of DMT was analyzed from the malformed sperm axoneme, and the comparison with the intact sperm axoneme DMT showed that the stability of A tubule in DMT was better than that of B tubule. B tubule was crushed in the process of "deformation", and most of the MIPs were lost, while A tubule had almost no morphological changes, and the MIPs of which were also consistent with the natural state, which also indicated that A tubule played a greater role in the stability of DMT than B tubule. These findings not only reveal the specialized structure of DMT in mammalian sperm, but also provide a structural basis for the treatment of human infertility caused by DMT defects.
In their 96nm DMT density map, it was possible to determine the densities of IDA, ODA, N-DRC, RS1, RS2, and RS3, which were also not available in single-particle data. Therefore, Cryo-FIB, Cryo-ET and STA have proved to be important research techniques for studying the complex structure of sperm axoneme in natural environments. These methods have great potential to advance our understanding of the mechanisms of sperm motility in mammals. Axoneme abnormalities are closely related to asthenospermia and infertility. Exploring the in-situ structure of sperm axoneme will shed new light on the underlying causes of these diseases.
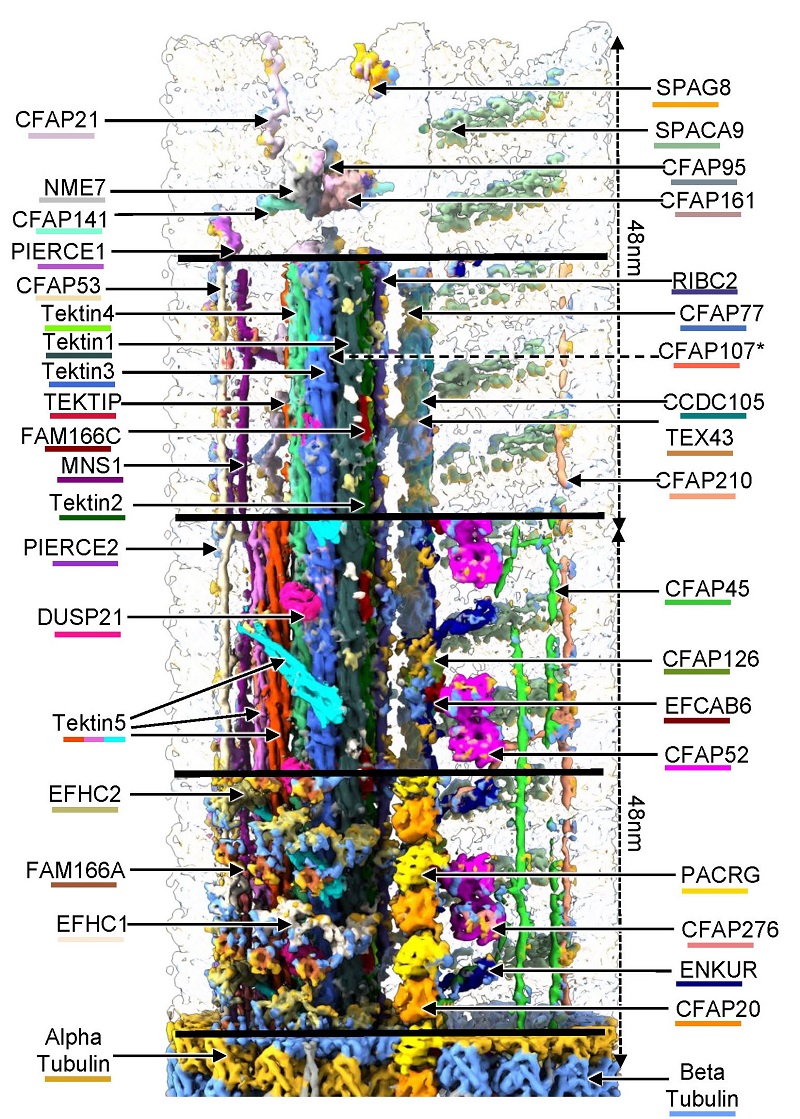
Figure 1. Schematic diagram of in situ structure of mouse sperm axoneme DMT
Article link: https://www.nature.com/articles/s41421-023-00606-3
Contact: ZHU Yun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhuyun@ibp.ac.cn
(Reported by Prof. SUN Fei’s group)

