Scientists decipher the molecular mechanisms underlying caspase-4 noncanonical inflammasome recognition and activation of inflammatory cytokine IL-18
On Nov 22th, 2023, Nature published online a research article entitled "Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome" by a collaborative study of Prof. DING Jingjin's group (Institute of Biophysics, Chinese Academy of Sciences) and Prof. SHAO Feng's group (National Institute of Biological Sciences, Beijing). This work identified inflammatory cytokine IL-18 as a physiological target of lipopolysaccharide-activated caspase-4 noncanonical inflammasome, and revealed the precise molecular mechanisms of IL-18 processing and maturation by inflammatory caspases in innate immunity.
Inflammasomes are critical components of innate immune system that coordinate autonomous intracellular defenses and induction of systemic inflammation. In the canonical inflammasome, a nucleotide-binding-oligomerization-domain-like receptor (NLR) senses pathogen products or endogenous dangers to activate caspase-1. The noncanonical inflammasome involves activation of mouse caspase-11 or its human orthologues caspases-4/5 that directly recognize bacterial lipopolysaccharide (LPS). Activated caspase-1 or caspase-4/5/11 cleave gasdermin D (GSDMD) to induce pyroptosis. Whereas caspase-1 processes IL-1β and IL-18 for maturation, no cytokine target has been firmly established for LPS-activated caspase-4/5/11.
Here we show that activated human caspase-4, but not mouse caspase-11, directly and efficiently processes IL-18 in vitro and during bacterial infections. Caspase-4 cleaves the same tetrapeptide site in pro-IL-18 as caspase-1. The crystal structure of the caspase-4-pro-IL-18 complex reveals a two-site (binary) substrate-recognition mechanism; the catalytic pocket engages the tetrapeptide, and a unique exosite that critically recognizes GSDMD similarly binds to a specific structure formed jointly by the propeptide and post-cleavage-site sequences in pro-IL-18.
This binary recognition is also used by caspase-5 as well as caspase-1 to process pro-IL-18. In caspase-11, a structural deviation around the exosite underlies its inability to target pro-IL-18, which is restored by rationally designed mutations. The structure of pro-IL-18 features autoinhibitory interactions between the propeptide and the post-cleavage-site region, preventing recognition by the IL-18Rα receptor. Cleavage by caspase-1, -4 or -5 induces substantial conformational changes of IL-18 to generate two critical receptor-binding sites.
Our study establishes IL-18 maturation as a cytokine response downstream of caspase-4 noncanonical inflammasome. This pathway, in contrast to canonical caspase-1-mediated IL-1β and IL-18 maturation that occurs mainly in monocytes, operates more prevalently in non-immune cells.
Released IL-18 stimulates IFNγ production in T cells and natural killer cells and promotes T helper 1 cell response. The caspase-4-IL-18 axis therefore bridges noncanonical inflammasome-activated pyroptosis and adaptive immunity.
IL-18 is a pleotropic cytokine and its dysregulation underlies various autoinflammatory or autoimmune diseases; IL-18-blocking antibodies have been tested in treating inflammatory bowel disease, atopic dermatitis and Bechet's disease. The finding of LPS-caspase-4/5-IL-18 axis also advances our understanding of the role of bacterial infection in IL-18-related diseases.
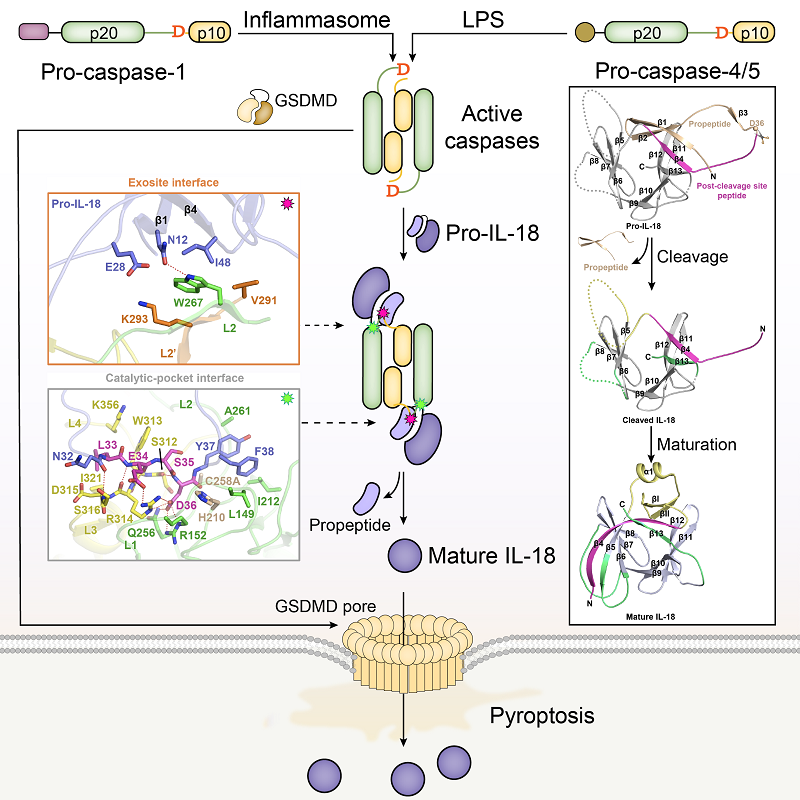
Figure: Schematic diagram of inflammatory cytokine IL-18 processing and maturation by inflammatory caspases in innate immunity
Prof. DING Jingjin from Institute of Biophysics, Chinese Academy of Sciences and Prof. SHAO Feng from National Institute of Biological Sciences are the co-corresponding authors of this work. Dr. SHI Xuyan from SHAO Feng's group and PhD student SUN Qichao from DING Jingjin's group are the co-first authors of this paper. This research was supported by the Strategic Priority Research Program of CAS, the Basic Science Center Project of NSFC, the National Key R&D Program of China, the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the CAS Youth Innovation Promotion Association, and the Tencent New Cornerstone Investigator Program.
Article link: https://www.nature.com/articles/s41586-023-06742-w
Contact: DING Jingjin
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: jding@ibp.ac.cn
(Reported by Prof. DING Jingjin’s group)

