Researchers Reveal Dual-function Mechanism of AcrIIA15
In response to the efficient attacks from the host CRISPR-Cas system, bacteriophages have evolved various resistance strategies, including encoding anti-CRISPR (Acr) proteins. Since their initial discovery in 2013, over a hundred Acr proteins have been identified. These proteins employ different mechanisms to inhibit the recognition and cleavage of target nucleic acids by the Cas protein-RNA complex, holding great potential for development as gene editing regulatory elements.
Recently, a research team led by Prof. WANG Yanli from the Institute of Biophysics, Chinese Academy of Sciences, published a research paper in Nature Communications, revealing the dual-function mechanism of the Staphylococcus phage-derived Acr protein AcrIIA15: it can inhibit the cleavage activity of the host CRISPR-Cas9 system, protecting the bacteriophage genome from destruction, and regulate the transcription of Acr itself to prevent a decrease in bacteriophage fitness due to excessive Acr expression.
AcrIIA15 contains two structural domains, the N-terminal and C-terminal domains. The researchers resolved the electron microscopy structure of the SaCas9, guide RNA, and AcrIIA15 CTD ternary complex, revealing that the AcrIIA15 surface exhibits strong negative charge, mimicking double-stranded DNA in both charge and shape to inhibit Cas9 from recognizing the target DNA.
Furthermore, AcrIIA15 also functions as a transcriptional repressor. By analyzing a series of crystal structures of AcrIIA15 with the inverted repeat sequence, it was found that NTD can mediate AcrIIA15 to form a dimer. Additionally, the researchers experimentally verified AcrIIA15's in vivo transcriptional repression function using fluorescent protein reporters.
The transcriptional repression regulation of Acr proteins is of significant importance for the complete life cycle of bacteriophages. This study ultimately resolved the electron microscopy structure of the AcrIIA15 dimer bound to both Cas9-sgRNA and the inverted repeat sequence, providing a panoramic view of AcrIIA15 achieving dual inhibition at the molecular level.
This work enhances our understanding of Acr inhibition mechanisms and provides structural biology support for the potential development of AcrIIA15 as a gene editing regulatory tool in the future.
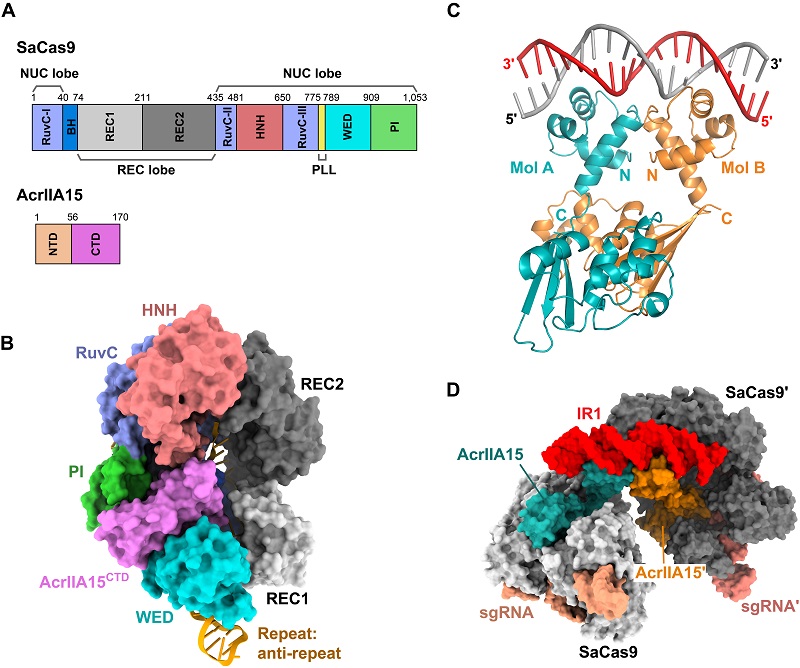
Figure. AcrIIA15 can inhibit the activity of Cas9 and regulate its own transcription
Article link:https://www.nature.com/articles/s41467-024-45987-5
Contact: SUN Wei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: sunwei@ibp.ac.cn
(Reported by Prof. WANG Yanli's group)

