Scientists Identify First Negative Regulator of NOX4 Translation
Nicotinamide adenine dinucleotide phosphate oxidase 4 (NADPH oxidase 4, NOX4) is a significant member of the NADPH oxidase family responsible for primarily producing H2O2. The regulation of NOX4 activity is predominantly through protein expression. However, the precise mechanisms by which highly secretory cells maintain the expression and activity of NOX4 while balancing H2O2 levels within the appropriate physiological range remain unclear.
On March 15, 2024, a research team headed by Prof. XU Pingyong from the Institute of Biophysics at the Chinese Academy of Sciences, released a study in Redox Biology introducing the first negative regulator of NOX4 translation, the pivotal factor EI24. They uncovered the molecular mechanism through which EI24 precisely governs H2O2 production by managing NOX4 translation, and its implications on upholding the redox equilibrium of pancreatic beta cells and insulin synthesis.
Researchers discovered that the endoplasmic reticulum-residing protein EI24 is responsive to fluctuations in H2O2 levels. Targeted deletion of the Ei24 gene in pancreatic beta cells notably elevated NOX4 protein expression and H2O2 levels within the endoplasmic reticulum.
Utilizing dual fluorescence reporter systems and immunoprecipitation assays, this research showcased how EI24 engages with the RNA-binding protein RTRAF, securing it to the 3'UTR region of Nox4 mRNA. This interaction inhibits the translation process, effectively controlling the overabundant generation of H2O2.
Deletion of EI24 prompted the translocation of RTRAF into the cell nucleus, releasing the inhibition on NOX4 translation, subsequently impacting the translation of the downstream transcription factor MAFA. Consequently, the knockout of Ei24 diminished the binding capacity of MAFA to the Ins2 promoter, hampering insulin production and perturbing blood glucose levels in mice.
This study unveils a novel co-translational regulatory system, elucidating how endoplasmic reticulum proteins precisely govern the co-translation of membrane-located proteins by modulating the localization of RNA-binding proteins. This regulatory process holds notable physiological significance and plays a critical role in sustaining the redox balance and vital functions of secretory cells.
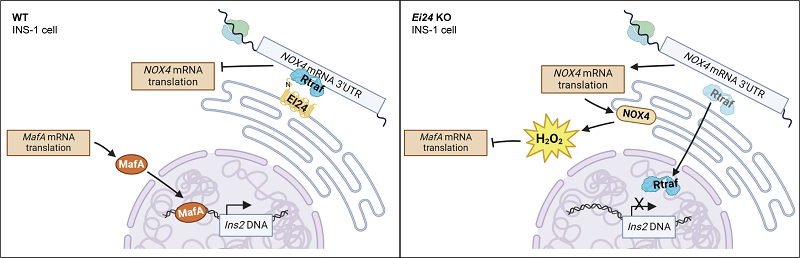
Fig. EI24 interacts with the RNA-binding protein RTRAF and Nox4 mRNA 3′-UTR to inhibit the translation of Nox4 in INS-1 cells
Article link: https://doi.org/10.1016/j.redox.2024.103126
Contact: XU Pingyong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: pyxu@ibp.ac.cn
(Reported by Prof. XU Pingyong's group)

