Scientists Resolve the Mystery of Lysosome Fission
Lysosomes are degradation centers and signaling hubs that play critical roles in cell and tissue homeostasis, development, and aging. Dysfunction of lysosomes is associated with occurrence and development of many kinds of diseases. To adapt to diverse cellular demands, lysosomes undergo constant fusion and fission to remodel their shape and functions. While the lysosome fusion process is extensively studied, little is known about lysosome fission. The molecules that execute scission of lysosomal membranes have not been revealed.
The research teams led by Prof. WANG Xiaochen and Prof. FENG Wei from the Institute of Biophysics, Chinese Academy of Sciences, have discovered that the HEAT-repeat protein HPO-27 is a long-sought lysosome fission factor. The study is published online in Nature on March 27.
The research team performed forward genetic screening in the multicellular organism C. elegans and identified the hpo-27 gene. hpo-27 encodes a conserved HEAT-repeat protein that is homologous to human MROH1. HPO-27 and MROH1 are large helical proteins containing 37 HEAT repeats, but their functions are unknown.
Using live-cell imaging, genetic analyses, and electron microscopy, the researchers found that loss of hpo-27 causes abundant lysosomal tubules in various tissues. In hypodermal cells, abundant lysosomal tubules form tubular networks, which fail to sustain and eventually collapse, causing severe lysosomal integrity defects. In agreement with the defects in morphology and integrity, lysosome function is impaired in hpo-27 mutants, leading to defective degradation of autophagic and phagocytic cargoes. hpo-27 mutants show severe developmental defects and shortened lifespan. This indicates that HPO-27 plays important roles in the maintenance of lysosome morphology, integrity, and functions, which is essential for animal development and longevity.
The researchers further investigated how C. elegans HPO-27 and human MROH1 regulate lysosome morphology and function. They performed a series of experiments in both worms and mammalian cells and found that 1) HPO-27 and MROH1 associate with lysosomes and concentrate at specific foci on the surface of lysosomes; 2) loss of HPO-27 causes a decrease in lysosomal fission and an increase in fusion, while overexpression of HPO-27 significantly reduces abundance of tubular lysosomes. In line with this, in MROH1 KO cells, the number of lysosome tubules reformed from autolysosomes is significantly increased, while tubule fission events are decreased. This indicates that HPO-27 and MROH1 may regulate lysosome fission. By both in vivo and in vitro assays, they found that HPO-27 and MROH1 are recruited to lysosomes as an effector of RAB7, a lysosomal small GTPase. Super-resolution time-lapse imaging revealed that HPO-27 and MROH1 are recruited to lysosomes and enriched at the fission sites to mediate membrane constriction and fission.
Next, the researchers investigated whether HPO-27 and MROH1 possess membrane fission activity using the supported membrane tube (SMrT) assay, which follows membrane fission in real time. They found that HPO-27 and MROH1 proteins purified from insect cells formed bright discrete foci along the length of the SMrTs and caused membrane constriction and scission of membrane tubes. By combinatory approaches of structural prediction, negative-staining EM, in vitro fission assay, they showed that HPO-27 and MROH1 self-assemble in a head-to-tail mode to mediate constriction and fission of lysosomes in vivo and supported membrane tubes in vitro. They further revealed that the terminal Heat repeat motif is required for self-assembly, which is essential for lysosomal association and membrane fission by HPO-27.
In summary, this study identifies HPO-27 and MROH1 as a long-sought lysosomal fission factor. Unlike the Dynamin superfamily proteins, which catalyze GTP hydrolysis for membrane scission, HPO-27 and MROH1 do not possess ATPase or GTPase activity. In in vitro fission assay, HPO-27 and MROH1 mediate SMrTs scission in the absence of ATP or GTP. Thus, HPO-27 and MROH1 are a new type of self-assembly scission factor. This work opens a new path to explore lysosome fission and perhaps other types of membrane fission processes.
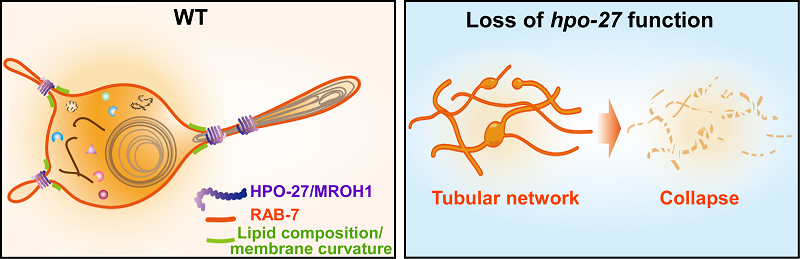
Figure. Proposed model of HPO-27 function in lysosomal fission
(IMage by WANG Xiaochen 's group)
Article link: https://www.nature.com/articles/s41586-024-07249-8
Contact: WANG Xiaochen
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: wangxiaochen@ibp.ac.cn
(Reported by Prof. WANG Xiaochen 's group)

