Researchers revealed novel mechanisms for cleavage-independent activation of gasdermins in ancient eukaryotes
Pyroptosis is a form of lytic cell death executed by a family of pore-forming proteins named gasdermin (GSDM). Pyroptosis plays crucial roles in host against pathogen infection and eliminating abnormal and harmful cells. Mammalian GSDMs, except for PJVK, feature an autoinhibited two-domain architecture, and are activated by protease cleavage within the linker between the two domains. The unleashed N-terminal effector domains of GSDMs assemble into oligomeric pores on plasma membranes, triggering pyroptosis. GSDMs are evolutionarily conserved and present in all kingdoms of life including prokaryotic bacteria. Proteolytic cleavage appears to be a universal mechanism for all GSDMs activation. Whether there exist activation mechanisms other than protease cleavage for GSDMs is unknown.
On Apr 25, 2024, the journal Science published online an article entitled "Cleavage-independent activation of ancient eukaryotic gasdermins and structural mechanisms" by a collaborative study of Prof. DING Jingjin's group (Institute of Biophysics, Chinese Academy of Sciences) and Prof. SHAO Feng's group (National Institute of Biological Sciences, Beijing). This work revealed two types of cleavage-independent mechanisms for GSDM activation in basal eukaryotes.
Extensive sequence searches identified a protein from the basal metazoan Trichoplax adhaerens, containing a single domain distantly related to GSDM-N domain. This protein (TrichoGSDM) existed in a monomer or a homodimer state. The monomer but not the dimer exhibited pore-forming activities on liposomes containing acidic phospholipids. Crystal structure of the dimer validates a GSDM-N-like fold and uncovers three inter-monomer disulfide bonds. Reduction of the disulfides by a reducing agent or the conserved antioxidant system (GSH or thioredoxin) generated pore-forming active monomers capable of killing bacteria. Cryo-EM structure illustrates a giant 44-mer TrichoGSDM pore and reveals conformational changes and the pore-assembly mechanism, akin to those of mammalian GSDMs.
The discovery of TrichoGSDM prompted the exploration of other pore-forming-domain-only GSDMs. A recent study reports that the filamentous fungus Neurospora crassa undergoes regulator of cell death-1 (rcd-1)-dependent cell death during allorecognition. We showed that RCD-1-1/RCD-1-2 were both monomers. Crystal structures demonstrate that the two highly homologous proteins fold similarly as mammalian GSDM-N domains and contain no autoinhibitory structural elements. RCD-1-1 and RCD-1-2 alone could bind phospholipids and be membrane associated but formed no pores. Co-existence of RCD-1-1 and RCD-1-2 readily formed pores and caused pyroptosis in bacterial, fungal and mammalian cells. Cryo-EM structure illustrates a pore of 11 RCD-1-1/RCD-1-2 heterodimers. The pore features two distinct inter-subunit interfaces, one of which mediates RCD-1-1/RCD-1-2 heterodimerization. The heterodimerization triggers conformational changes, leading to their oligomerization into membrane-inserted pores. The mechanism was validated by structure-guided mutagenesis analyses in yeast and N. crassa fusion assays. Further analyses explain why homogenous RCD-1, though being membrane-associated, is pore-formation incompetent.
Thus, TrichoGSDM and RCD-1 represent two types of pore-forming-domain-only GSDMs, which come from simple and ancient eukaryotes and employ distinct cleavage-independent activation mechanisms. TrichoGSDM is a disulfides-linked autoinhibited dimer and activated by reduction of the disulfides, indicating a redox-responsive function. The pore-forming activity in RCD-1 is stimulated by hetero-recognition between RCD-1-1 and RCD-1-2 from genetically incomparable fungal strains, underlying allorecognition-caused cell death in N. crassa. The findings highlight mechanistic diversities in GSDM activation as well as versatile biological functions in the emerging GSDM family.
Prof. DING Jingjin from Institute of Biophysics, Chinese Academy of Sciences and Prof. SHAO Feng from National Institute of Biological Sciences are the co-corresponding authors of this work. PhD student LI Yueyue, Dr. HOU Yanjie and Dr. SUN Qi are the co-first authors of this paper. This research was supported by the National Key R&D Program of China, the Strategic Priority Research Program of CAS, the Basic Science Center Project of NSFC, the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the CAS Youth Innovation Promotion Association, and the Tencent New Cornerstone Investigator Program.
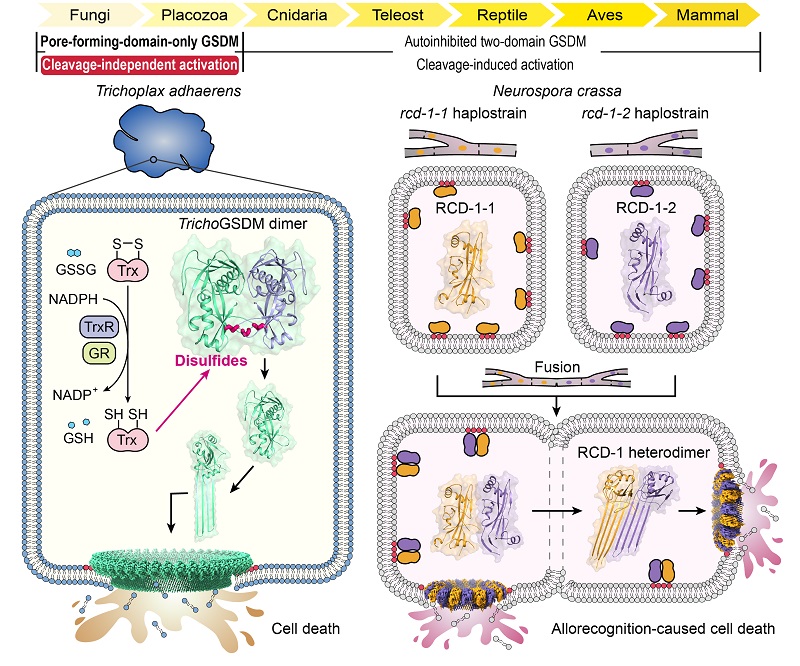
Figure: Basal eukaryotes Trichoplax adhaerens and Neurospora crassa both harbor pore-forming-domain-only GSDMs that are activated to cause cell death through cleavage-independent mechanisms.
(Image by DING Jingjin's group)
Article link: https://www.science.org/doi/10.1126/science.adm9190
Contact: DING Jingjin
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: jding@ibp.ac.cn
(Reported by Prof. DING Jingjin's group)

