Scientists Reveal Agonist Recognition Mechanism of SUCR1
The succinate receptor SUCR1 is a potential therapeutic target for ulcerative colitis, liver fibrosis, diabetes, and rheumatoid arthritis. It plays a critical role in regulating mitochondrial reactive oxygen species homeostasis. Due to the lack of human SUCR1 structure, the mechanisms of succinate (SA) perception, receptor activation, and signal transduction by SUCR1 are still unknown.
On May 14, 2024, a paper published in Cell Research by Prof. WANG Jiangyun's team from the Institute of Biophysics, Chinese Academy of Sciences, reported a progress in the structural basis research of the succinate-activated human succinate receptor SUCR1.
This study overcame a series of challenges such as low ligand binding affinity and complex assembly difficulties, and successfully resolved the structures of succinate bound SUCR1-Gq and cis-epoxy succinate (ESA) bound SUCR1-Gi complexes using the single-particle cryo-electron microscopy (cryo-EM) technology.
Researchers found that the extracellular loop 2 (ECL2) and the residue R993.29 in SUCR1 activation play key roles. The precise positioning of ECL2 is necessary for the formation of the functional agonist binding site.
Furthermore, structural comparisons of SUCR1-Gq and SUCR1-Gi revealed that the α5-helix of Gi and Gq displayed different orientations in the intracellular cavity of SUCR1, resulting in distinct interaction barcodes between SUCR1 and Gq or Gi proteins, explaining the mechanism of SUCR1's coupling selectivity with downstream G proteins.
This research elucidates the molecular activation mechanism and G protein selectivity mechanism of SUCR1, which will promote the exploration of chemical sensing and drug design targeting succinate receptors.
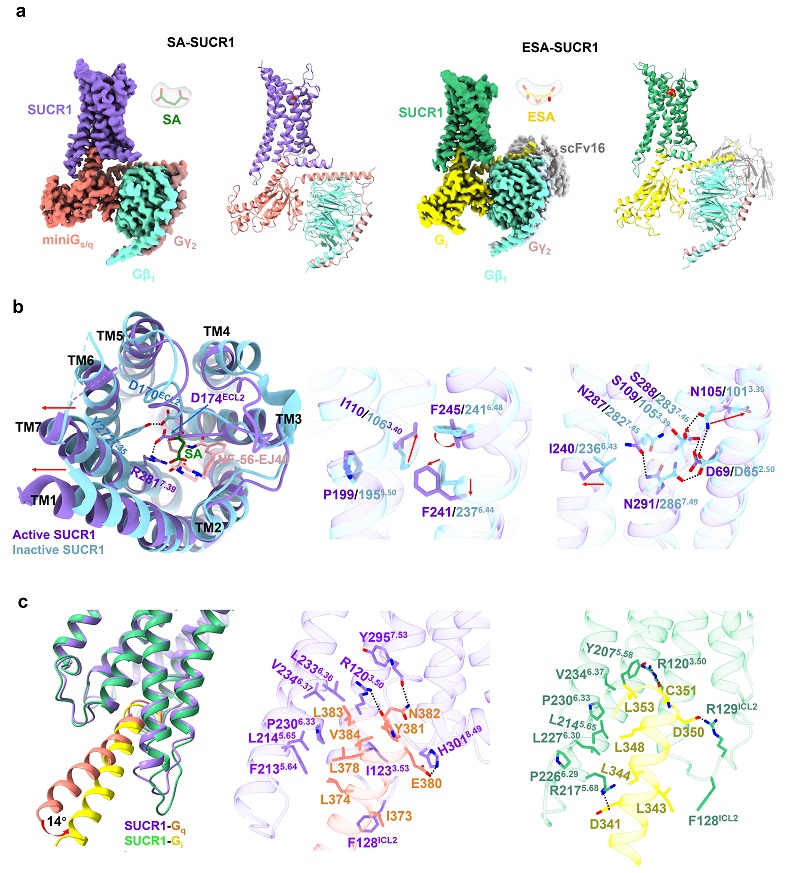
Fig. The molecular activation and G protein selectivity mechanism of the succinate receptor
(Image by WANG Jiangyun's group)
Article link: https://www.nature.com/articles/s41422-024-00968-7
Contact: LI Fahui
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: lifahui@ibp.ac.cn
(Reported by Prof. WANG Jiangyun's group)

