Researchers Unveil Structural Mechanism of RhoBAST Binding and Activating Fluorescent Dye TMR-DN
RhoBAST, one of the fluorescent light-up RNA aptamers (FLAPs), can bind and activate a contact-quenched type of rhodamine derivative TMR-DN (a coupled compound of the fluorescent group TMR and the quencher DN). Due to its excellent folding performance, high affinity, rapid ligand exchange, ultra-brightness, outstanding photostability, and other characteristics, RhoBAST exhibits excellent performance in super-resolution imaging of RNA. However, the structural mechanism of RhoBAST binding and activating TMR-DN is not yet clear.
On May 17, 2024, a research group led by Prof. FANG Xianyang from the Institute of Biophysics of the Chinese Academy of Sciences and Prof. LI Xing from the Institute of Zoology collaborated to publish a research paper in Nature Communications. In this study, researchers revealed the structure of RhoBAST and its molecular mechanism of binding and activating TMR-DN by integrating X-ray crystallography, small-angle X-ray scattering, molecular dynamics simulations, and other methods.
Researchers first used X-ray crystallography to resolve the high-resolution structure of the RhoBAST-TMR-DN complex, finding that RhoBAST folds into a four-way junction structure, with an overall structure resembling an asymmetric "A" shape.
Small-angle X-ray scattering experiments indicated that the folding of RhoBAST does not depend on ligand molecules, and this structural rigidity allows it to avoid photobleaching issues by rapidly exchanging with fresh ligands in the solution, thereby exhibiting excellent optical properties in fluorescence super-resolution imaging.
Researchers characterized the conformational space of TMR-DN in both free and bound states with RhoBAST using enhanced sampling molecular dynamics simulations. They found that, whether in the free or bound state, TMR-DN displays highly dynamic conformational features, with the contact-but-not-stached conformation being prevalent.
By integrating multiple research methods, this work elucidated the structure of RhoBAST and its mechanism of binding and activating the contact-quenched type of fluorophore TMR-DN, further providing mechanistic insights for the rational design and optimization of this important FLAP system through comparing the structures and binding modes of related FLAPs.
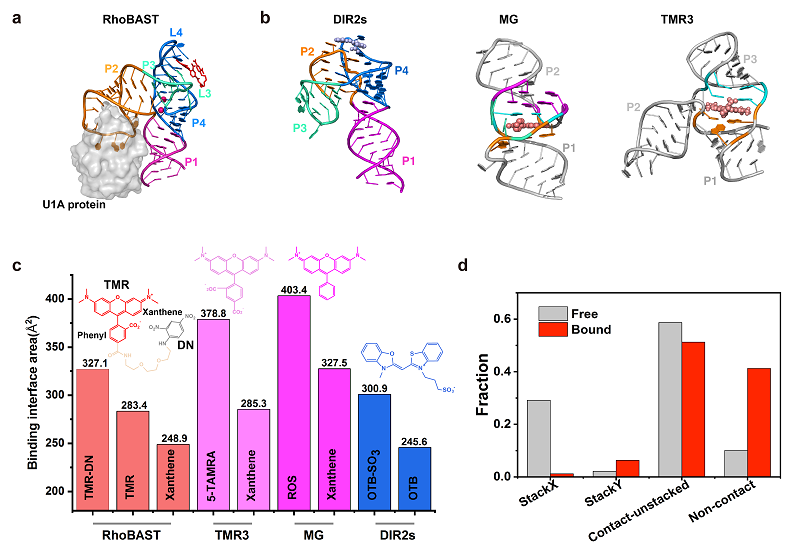
Fig. (a) A schematic diagram of the crystal structure of the RHOBAST-TMR-DN complex; (b-c) Comparison of structures and binding areas of other representative fluorescent RNA aptamer complexes; (d) Statistical analysis of different conformations before and after the binding of TMR-DN to RhoBAST.
(Image by FANG Xianyang's group)
Article link: https://doi.org/10.1038/s41467-024-48478-9
Contact: Fang Xianyang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: fangxy@ibp.ac.cn
(Reported by Prof. FANG Xianyang's group)

