A Brain Insulin Signal Encodes Protein Satiety and Suppresses Feeding
Insulin is a highly conserved hormone in evolution, playing a key role in various biological processes such as growth, development, and metabolism. In general, insulin signals represent high nutrition levels and participate in the regulation of feeding behavior. However, it is not clear whether insulin signals can encode specific types of nutritional information and directly regulate feeding behavior by transmitting signals to specific neurons.
On May 24, 2024, a research group led by Prof. LI Yan at the Institute of Biophysics, Chinese Academy of Sciences, published a research paper in Cell Reports, reporting a brain insulin signal that encodes protein satiety and its neural mechanism in regulating protein feeding behavior, revealing a new function of insulin signaling in the central nervous system.
The researchers conducted behavioral screening by knocking down insulin receptors and identified the direct downstream target of brain insulin-producing cells (IPCs) - T1 dopamine neurons. Following protein satiety, the activity of T1 neurons significantly increased. Activating T1 neurons or increasing insulin signaling within them specifically inhibited protein feeding in flies, while inhibiting T1 neurons or reducing insulin signaling eliminated the feeding suppression effect after protein satiety.
Interestingly, activating IPCs led to a decrease in protein and sugar intake in flies, while inhibiting T1 neurons selectively blocked the decrease in protein intake without affecting the decrease in sugar intake. This indicates that insulin signaling can represent satiety information for various types of nutrients, with T1 neurons specifically mediating protein satiety information.
Through approaches such as EM connectome mapping and in vivo calcium imaging, the researchers discovered downstream of T1 neurons, namely PB-LNs. Similar to T1 neurons, inhibiting PB-LNs also blocked the feeding suppression effect after protein satiety. However, activating PB-LNs no longer showed nutrient selectivity in inhibiting feeding behavior, indicating the integration of nutritional information of protein food.
This study demonstrates that insulin signaling in the central nervous system can encode high protein nutritional information and participate in immediate and precise behavioral regulation through specific downstream neurons. In mammals, there are various insulin and insulin-like signals in the brain. These findings in flies provide important insights for studying the functions of insulin signals in the central nervous system of mammals and its mechanisms in regulating feeding behavior.
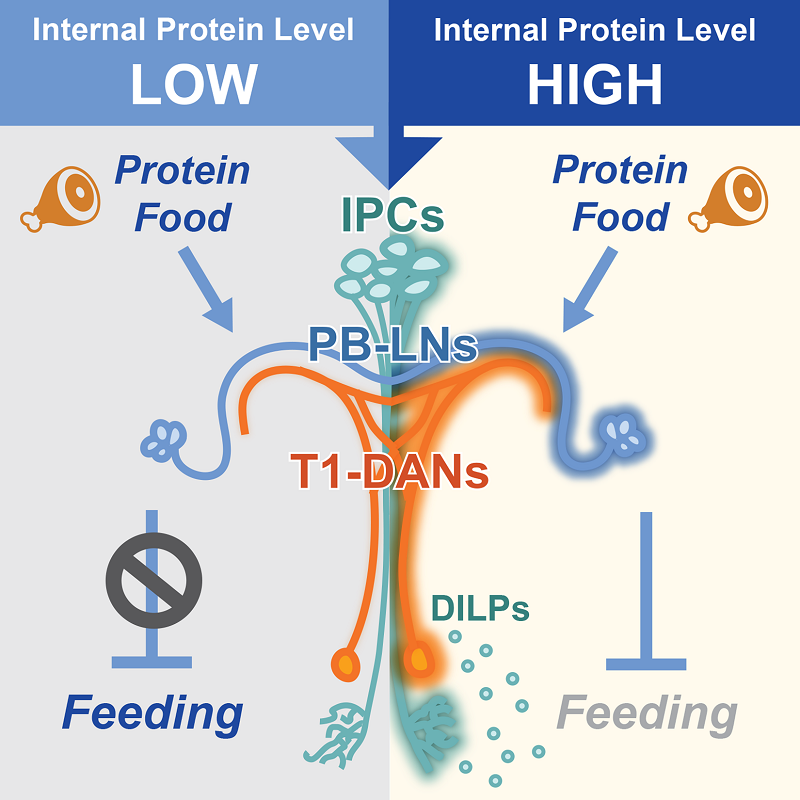
Fig. Neural circuitry regulating feeding behavior through a brain-derived insulin signal encoding protein satiety
(Image by LI Yan's group)
Article link: https://doi.org/10.1016/j.celrep.2024.114282
Contact: LI Yan
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: liyan@ibp.ac.cn
(Reported by Prof. LI Yan's group)

