PSII-TPP Complex: Hunting for Key Molecules in the Early Stage of Photosystem II Repair Cycle in Green Algae
Photosystem II (PSII) is the energy converter in the oxygenic photosynthetic system. The functional groups in PSII are highly susceptible to damage under light induction and by the action of reactive oxygen species generated during photosynthesis, leading to loss of activity. Damaged PSII needs to have its function restored through a complex biological process known as the PSII repair cycle, the molecular mechanisms of which are elusive and remain to be explored in depth.
On June 18, 2024, a research group led by Prof. LIU Zhenfeng from the Institute of Biophysics, Chinese Academy of Sciences, in collaboration with the research groups of Prof. LI Xiaobo from Westlake University and Prof. TIAN Lijin from the Institute of Botany, Chinese Academy of Sciences, published a research paper in Nature Communications.
The researchers applied a combination of biochemical, mass spectrometry analysis, and cryo-electron microscopy techniques to discover a protein complex consisting of TEF14 (Thylakoid Enriched Factor 14), PRF1 (Photosystem II Repair Factor 1), PRF2 (Photosystem II Repair Factor 2), and an α-tocopherol quinone molecule (α-TQ) bound to the monomeric PSII core complex prepared under high light conditions, thus naming it the PSII-TPP complex (Figure 1).
TEF14 is a soluble protein that binds to the luminal domain surface of the CP47 subunit, a core antenna complex of the PSII core. Studies suggest that TEF14 may be involved in the dissociation process of PsbO during the disassembly of the PSII supercomplex in the repair process.
PRF1 is a single-pass transmembrane protein, and the study results indicate that this protein may be involved in promoting the dissociation of a peripheral antenna complex named CP29 from PSII during the PSII repair process.
PRF2 is another single-pass transmembrane protein located near cytochrome b559, and its binding causes the plastoquinone diffusion channel in PSII toswitch from an open to closed state.
α-Tocopherol quinone (α-TQ) is a small molecule compound that plays a crucial role in plant photoprotection processes. This work discovered, for the first time, the α-TQ binding site near cytochrome b559, suggesting that α-TQ may prevent the transfer of electrons from cytochrome b559 to surrounding oxygen molecules through competitive oxidation, thereby preventing the generation of reactive oxygen species and protecting PSII.
Based on biochemical and structural analysis results, this study proposes a model for the functional mechanisms of TEF14, PRF1, PRF2, and α-TQ in the PSII repair cycle (Figure 2).
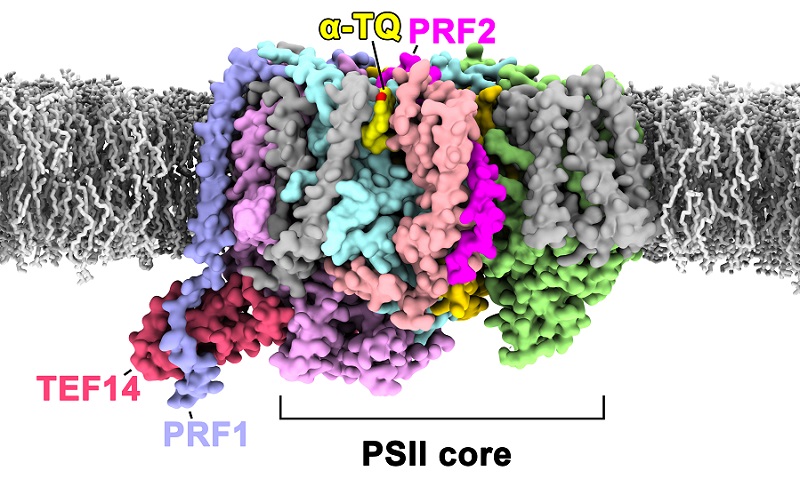
Fig. 1 PSII-TPP complex formed during the early stages of PSII repair
(Image by LIU Zhenfeng's group)
The PSII core large subunit, along with the newly discovered three protein factors and α-TQ molecule, are shown using a colored surface model, while some other small subunits are represented by a gray surface model. Lipid molecules on the thylakoid membrane surrounding the PSII-TPP complex are depicted using a gray stick model. The thylakoid membrane in the figure is a predicted model used to demonstrate the relative relationship between PSII-TPP and the membrane.
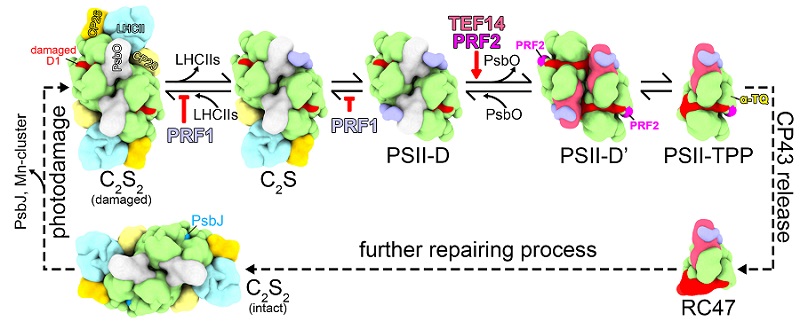
Fig. 2 Model of the functional mechanisms of TEF14, PRF1, PRF2, and α-TQ in the early stages of PSII repair cycle
(Image by LIU Zhenfeng's group)
Article link: https://doi.org/10.1038/s41467-024-49532-2
Contact: LIU Zhenfeng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: liuzf@ibp.ac.cn
(Reported by Prof. LIU Zhenfeng's group)

