AcrIF25 Inhibits Activity of CRISPR-Cas System
On July 3, 2024, a research paper was published in Nature by a collaborative effort between Dr. WANG Yanli's research team at the Institute of Biophysics, Chinese Academy of Sciences, and Dr. Alan Davidson's research team at the University of Toronto, Canada. They discovered a novel anti-CRISPR protein called AcrIF25, which inhibits the activity of the CRISPR-Cas system by disassembling the I-F type CRISPR-Cas complex (Csy complex), providing a new approach for precise control of the CRISPR-Cas system.
The CRISPR-Cas system is a powerful gene-editing tool widely present in bacteria and archaea used for defending against the invasion of foreign DNA. To counteract this defense system, some phages have evolved proteins capable of inhibiting the activity of the CRISPR-Cas system, known as anti-CRISPR proteins (Acr).
In this study, researchers first identified a new anti-CRISPR protein, named AcrIF25, using bioinformatics. The in vivo assays demonstrated that AcrIF25 significantly inhibits the activity of the I-F type CRISPR-Cas system of Pseudomonas aeruginosa.
Size-exclusion chromatography results showed that AcrIF25 does not directly bind to the complete Csy complex but specifically interacts with the Cas7 subunits and dissociates them from the Csy complex, thereby blocking the function of the CRISPR-Cas system.
To further elucidate the mechanism of AcrIF25, researchers solved the crystal structures of AcrIF25 and the Cas7:AcrIF25 complex. They found that AcrIF25 interacts with Cas7 through its C-terminal domain, covering the binding interface between Cas7 subunits and the interface between Cas7 and crRNA. Therefore, the binding of AcrIF25 disrupts Cas7 interacting with adjacent Cas7 subunits and crRNA, leading to the disassembly of the entire Csy complex.
Notably, AcrIF25 can remove Cas7 subunits from the Csy complex without using energy derived from ATP hydrolysis. AcrIF25 is the first example of a protein that disassembles a large and stable protein-nucleic acid complex in the absence of external energy supply.
This study not only enriches our understanding of the mechanism of Acr proteins but also provides valuable clues for the development of new gene-editing tools in the future.
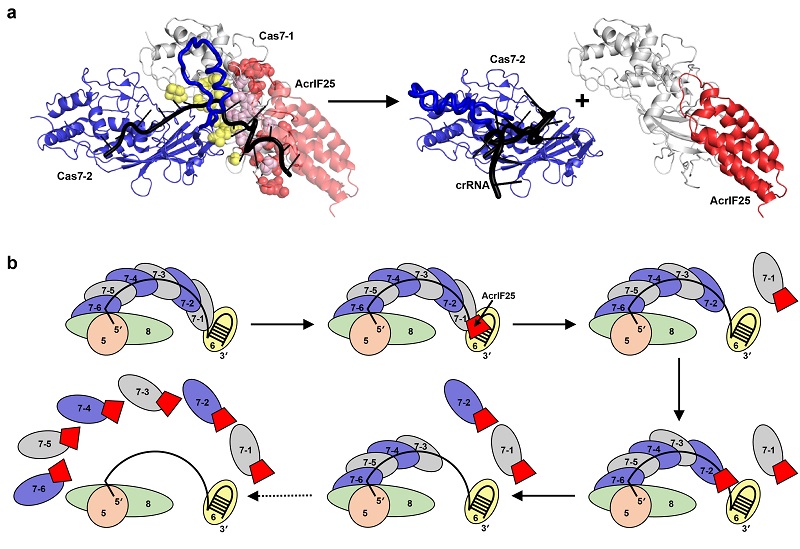
Fig. The molecular mechanism of AcrIF25 dissociating the Csy complex
(Image by Wang Yanli's group)
Article link: https://www.nature.com/articles/s41586-024-07642-3
Contact: WANG Yanli
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: ylwang@ibp.ac.cn
(Reported by Prof. WANG Yanli's group)

