Scientists Discover How S-nitrosoglutathione Reductase (GSNOR) Regulates Morphine Analgesic Tolerance
Recently, a collaborative research team led by Prof. CHEN Chang from the Institute of Biophysics, CAS and Prof. YAO Yonggang from the Kunming Institute of Zoology, CAS, utilized genetically modified mice lacking the S-nitrosoglutathione reductase (GSNOR) gene and mice overexpressing GSNOR in neurons to establish a morphine-induced analgesic tolerance mouse model. It elucidated that GSNOR regulates the S-nitrosation of protein kinase C-alpha (PKCα), affecting PKCα kinase activity, and thereby participating in morphine analgesic tolerance. Their findings were published in Redox Biology.
Morphine is the most effective and widely used analgesic in clinical practice. Prolonged use of morphine can lead to tolerance and addictive side effects, but the mechanism of morphine analgesic tolerance is not yet fully understood.
Regarding the role of GSNOR in morphine analgesic tolerance, researchers conducted systematic collaborative research at various levels, including molecular, cellular, and mouse models. They found that chronic morphine injection in mice significantly reduced GSNOR in the cerebral cortex and increased the total protein S-nitrosation levels.
Through quantitative proteomic analysis of S-nitrosation, researchers discovered that GSNOR regulates the S-nitrosation levels of the PKCα protein during morphine analgesic tolerance. Mouse model experiments confirmed that morphine injection increased the S-nitrosation levels of PKCα in the cerebral cortex.
Further research showed that GSNOR can regulate the S-nitrosation of PKCα protein at the Cys78, Cys86, and Cys132 sites. After S-nitrosation modification of PKCα, its kinase activity is inhibited, thereby participating in morphine-induced analgesic tolerance. Pre-injection of GSNOR inhibitor N6022 or PKCα inhibitor G06976 in mice promoted morphine-induced analgesic tolerance behavior.
Various experimental evidence suggests that GSNOR plays a significant role in morphine analgesic tolerance, revealing a new mechanism in which protein S-nitrosation modification regulates morphine analgesic tolerance.
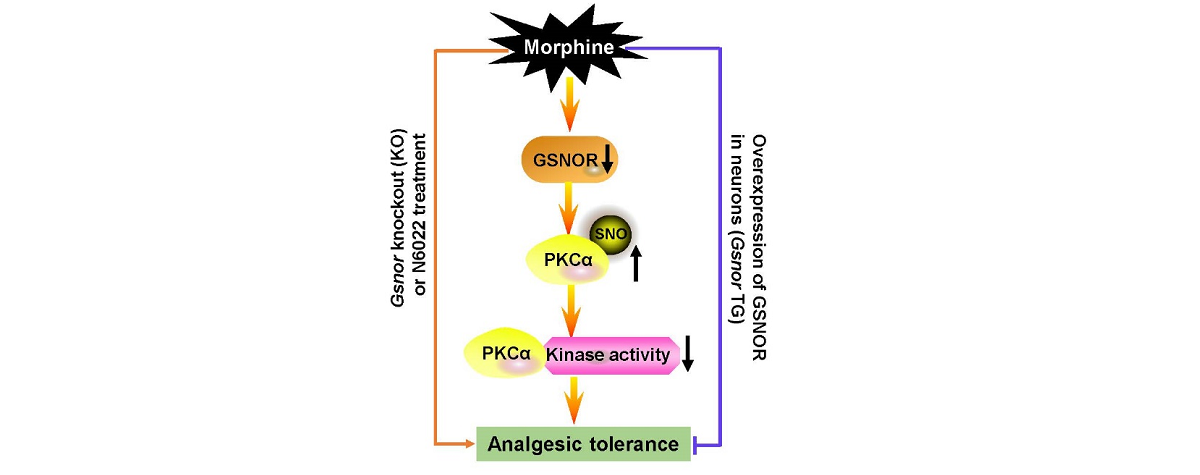
Fig. Schematic of potential mechanism underlying the effects of GSNOR on morphine analgesic tolerance
(Image by CHEN Chang's group)
Article link: https://www.sciencedirect.com/science/article/pii/S2213231724002179
Contact: CHEN Chang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: chenchang@ibp.ac.cn
(Reported by Prof. CHEN Chang's group)

