Researchers Reveal the Transport Mechanism of Noradrenaline Transporter and Binding Modes of Small-Molecule and Peptide Drugs
Noradrenaline (NA) is an important monoamine neurotransmitter in the nervous system. The noradrenergic pathway in the brain originates from the locus coeruleus (LC) and projects diffusely to a variety of forebrain targets, regulating various neural activities such as mood, attention, memory, and libido. Additionally, NA can bind to α2A receptors on nociceptive afferent neurons in the dorsal root ganglion, inhibiting the transmission of pain signals. In noradrenergic neurons, NA is released from the presynaptic membrane into the synaptic cleft, where it activates noradrenaline receptors, initiating a series of signaling processes. The noradrenaline transporter (NET), located on the presynaptic membrane, utilizing the Na+ gradient to couple 'downhill' transport of Na+ with 'uphill' transport of their substrates across the plasma membrane, thereby terminating the neural signal transmission. The role of NET in NA reuptake maintains the balance of NA levels in the nervous system, enabling the normal physiological functioning of the nervous system. Currently, various clinically-used small molecule drugs inhibit NET to increase NA concentration in the synaptic cleft, thereby enhancing the signaling of noradrenergic neurons to treat diseases such as depression and attention-deficit hyperactivity disorder (ADHD). Furthermore, the peptide toxin χ-MrlA can specifically inhibit NET in a non-competitive manner, blocking pain signal transmission by enhancing α2A receptor activity on nociceptive afferent neurons, demonstrating significant analgesic effects. However, the transport substrate and binding modes of different drug molecules with NET remain unclear.
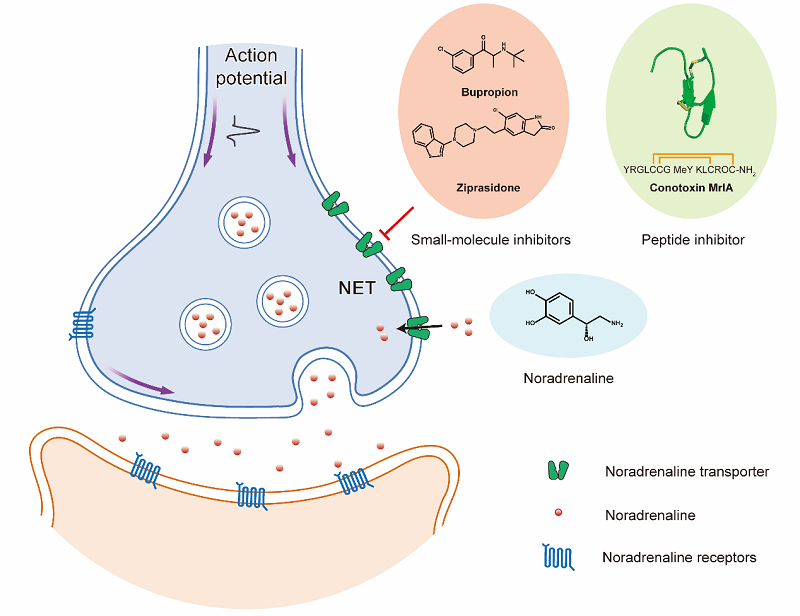
Figure 1. Noradrenergic Neuron
(Image by ZHAO Yan's group)
On July 31, 2024, ZHAO Yan's research group from the Institute of Biophysics, Chinese Academy of Sciences, in collaboration with Claus Loland's team from the University of Copenhagen, Denmark, published a research paper in Nature titled "Transport and inhibition mechanisms of the human noradrenaline transporter." The researchers used single-particle cryo-electron microscopy to resolve the high-resolution structures of the human noradrenaline transporter (NET) in various states: apo state (NETapo), bound with substrate noradrenaline (NETNA), bound with the conotoxin χ-MrlA analog (NETMrlA), bound with bupropion (NETBPP), and bound with ziprasidone (NETZPD). These complex structures reveal the structural characteristics of NET in multiple functional states and the binding modes of different drug molecules, deeply elucidating the transport mechanism of NET and providing a structural basis for developing new drugs.
Researchers resolved the structures of NETapo and NETNA in an inward-open state, both with a resolution of 2.6 Å. The substrate-bound complex structure revealed the substrate NA at the central binding site. Meanwhile, they unexpectedly discovered an additional conformation-specific substrate binding site on the extracellular side, which only exists in the inward-open state and disappears in the outward-open state due to local conformational changes. Mutating the key residues interacting at this site reduced transport activity of NET, suggesting this site might regulate transport function. Furthermore, NET is a secondary active transporter dependent on sodium and chloride ions. Researchers resolved the ion binding sites of NET in different transport functional states, providing a structural basis for understanding the coupling mechanism of ion and substrate binding and conformational changes.
χ-MrlA is a 13-amino acid peptide toxin extracted from cone snail Conus marmoreus and is the only known peptide inhibitor in the SLC6 family. Based on χ-MrlA, Xenome developed the analog XEN2174 for treating postoperative pain, cancer pain, and chronic pain. By resolving the complex structure with χ-MrlA, researchers found that χ-MrlA analogs stabilize NET in an outward-open conformation, deeply binding in the extracellular pocket of NET, forming extensive interactions. Comparing the substrate-bound complex structure, they found that χ-MrlA's binding site does not overlap with the substrate molecule, structurally explaining why χ-MrlA is a non-competitive inhibitor of NET. Combining structural analysis and functional experiments with mutants, researchers also revealed the key structural basis for χ-MrlA's selective inhibition of NET but not other transporters in this family such as the serotonin transporter (SERT) and dopamine transporter (DAT). This information provides an important structural basis for researching new peptide drugs targeting the monoamine transporter family.
Common clinically used antidepressants include selective serotonin reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors (SNRIs) and tricyclic inhibitors. These drugs mainly treat depression by inhibiting SERT but sometimes cause side effects such as nausea, headache, insomnia, and dry mouth. Bupropion is the only clinically antidepressant that selective inhibit NET and DAT, avoiding side effects caused by SERT inhibition. Previous studies found that the antipsychotic drug ziprasidone could simultaneously inhibit NET, DAT, and SERT. To reveal the molecular basis accounting for the selective inhibition of monoamine transporters, researchers resolved the complexes of NET with bupropion and ziprasidone. Structural analysis revealed that, unlike classic antidepressants, bupropion and ziprasidone both bind in the inward-facing binding pocket of NET. Comparing the binding modes of the two drug molecules, researchers found that the tert-butyl group of bupropion and the ethyl group of ziprasidone are located in the bottleneck between TM6 and TM8. In contrast, the tert-butyl group of bupropion is larger than the ethyl group of ziprasidone. In SERT, the bottleneck becomes narrower due to the change from glycine to alanine on one side of the bottleneck, preventing bupropion from effectively inhibiting SERT, but this change does not affect ziprasidone's inhibition. These findings provide key information for developing drugs targeting monoamine transporters.
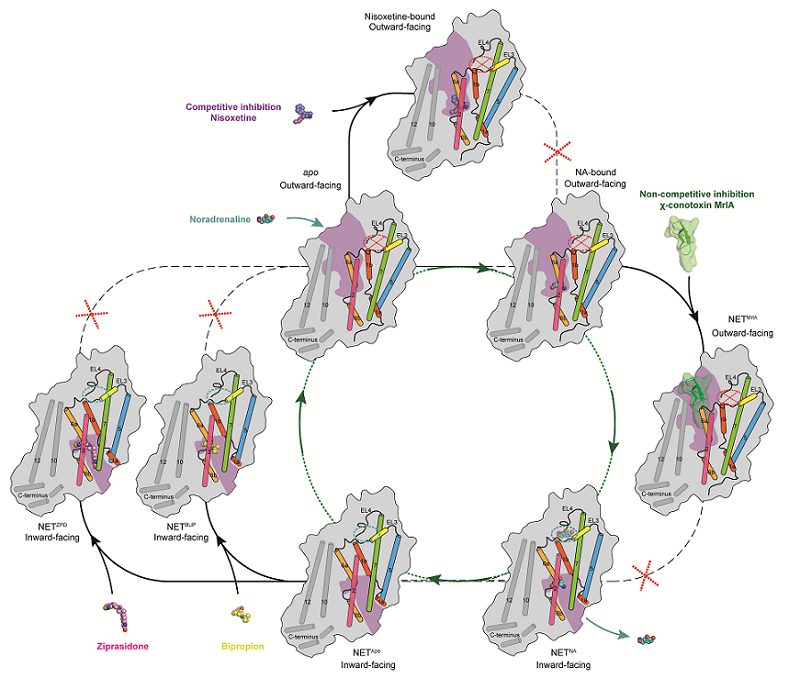
Figure 2. The transport cycle and inhibition modes of NET
(Image by ZHAO Yan's group)
Article link: https://www.nature.com/articles/s41586-024-07638-z
Contact: ZHAO Yan
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: zhaoy@ibp.ac.cn
(Reported by Prof. ZHAO Yan 's group)

