Scientists unveil the structural basis of catalytic selectivity of glycosyltransferase SgUGT94-289-3 towards variable mogrosides
On July 30, 2024, the research groups of LI Mei from the Institute of Biophysics, Chinese Academy of Sciences, and MA Xiaojun/LUO Zuliang from the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, published an article in the international journal Nature Communications. This study unveiled the structural basis of the catalytic selectivity of glycosyltransferase SgUGT94-289-3 towards mogrosides.
Mogrosides are a class of triterpene saponin secondary metabolites derived from the medicinal plant Siraitia grosvenorii (Luo Han Guo), exhibiting high sweetness and low calorie, with Mogroside V (M5) and Siamenoside I (SIA) being the major sweetness components. The production of M5 and SIA requires serial glucosylation events in mogrosides, and the glycosyltransferase SgUGT94-289-3 is a key enzyme in their biosynthesis. SgUGT94-289-3 continuously catalyzes glucosylation on mogroside IIe (M2E) and on the subsequent intermediate mogroside products, responsible for the biosynthesis of M5 and SIA. SgUGT94-289-3 recognizes a number of mogrosides as sugar acceptors, attaching glucose groups to both reactive ends of these mogroside substrates. However, the mechanism of its promiscuous substrate recognition and multiple catalytic modes remains unclear.
The research groups of LI Mei and MA Xiaojun/LUO Zuliang reported the structural and enzymatic characterization of SgUGT94-289-3. They discovered that SgUGT94-289-3 adopts a dual-pocket organization at its active site (Figure 1a). This configuration allows the two structurally distinct reactive ends of mogrosides to be presented from different pockets to the active site for glucosylation reaction, thus enabling both substrate promiscuity and catalytic regioselectivity. They identified a structural motif critical for catalytic activity and regioselectivity, and generated SgUGT94-289-3 mutants with greatly improved M5/SIA production based on their structural findings (Figure 1b).
This study reveals the dual-pocket substrate binding mode of SgUGT94-289-3, greatly advancing our understanding of the molecular mechanisms of the promiscuous recognition of asymmetric substrates by glycosyltransferases. The structural and functional characterization of the enzyme establish a foundation for designing SgUGT94-289-3 variants with enhanced catalytic activity and selectivity, and provides valuable guidance for developing engineering enzymes to catalyze multi-step reactions by a single enzyme.
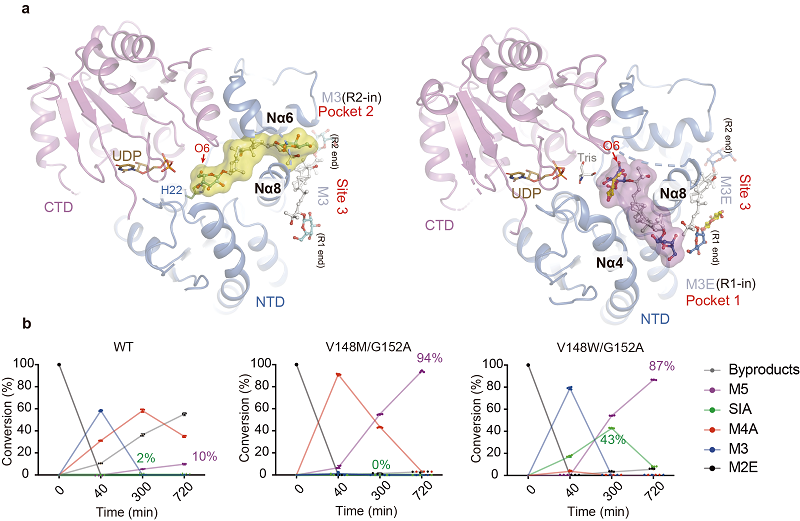
Figure 1. (a) Structural model of SgUGT94-289-3 with mogroside substrates (M3/M3E) bound in two distinct substrate pockets. (b) Conversion rates of various products during the continuous glycosylation reaction catalyzed by SgUGT94-289-3 mutants.
(Image by LI Mei's group)
Article link: https://doi.org/10.1038/s41467-024-50662-w
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: meili@ibp.ac.cn
(Report by Prof. LI Mei's group)

