Scientists found protein functions as toxin and antidote with its dual structures
November 1, 2024, the research teams led by Prof. YE Keqiong at the Institute of Biophysics, Chinese Academy of Sciences, and Prof. DU Lilin at the National Institute of Biological Sciences (NIBS), Beijing, published two related papers in "Proc. Natl. Acad. Sci. USA" entitled "A meiotic driver hijacks an epigenetic reader to disrupt mitosis in noncarrier offspring" and "Structural duality enables a single protein to act as a toxin-antidote pair for meiotic drive". The study found a new selfish genetic element in the fission yeast (Schizosaccharomyces pombe) and revealed the molecular and structure mechanism of its protein product in controlling cell survivial.
Killer meiotic drivers (KMDs) are selfish genetic elements that selective kill meiotic products lacking the KMD element, thereby promoting their own propagation. The study found that tdk1 is a new type of KMD in fission yeast. KMDs commonly achieve selective killing through separate toxins and antidotes. However, the single protein product of TDK1 can act as both a toxin and an antidote through structural changes, illustrating a new type of mechanism.
S. pombe primarily reproduces asexually as haploid cells but can undergo sexual reproduction upon starvation. During sexual reproduction, haploid cells of opposite mating types fuse to form diploids, which then undergo meiosis to produce four haploid spores. During vegetative growth and meiosis, Tdk1 exists in a tetrameric, non-toxic form. In spores, the C-terminal region of Tdk1 assembles into a toxic hexameric structure that binds to the acetylated histone reader Bdf1. Furthermore, the toxic Tdk1 protein forms supramolecular assemblies that could block chromosome segregation in mitosis in non-carrier offspring. During spore germination, newly synthesized Tdk1 dismantles these toxic assemblies, acting as an antidote to protect carriers.
In sum, this work found a new selfish killer gene and revealed the molecular and structural mechanism underling a single protein employing structural duality to form a toxin-antidote pair.
This research was a collaboration between the Institute of Biophysics, Chinese Academy of Sciences, and the National Institute of Biological Sciences, Beijing. Prof. YE Keqiong, Prof. DU Lilin, and Dr. HUA Yu served as the co-corresponding authors. Dr. HUA Yu, Dr. ZHANG Jianxiu, YANG Manyun and ZHANG Fanyi were the co-first authors. The research was supported by National Natural Science Foundation of China, Chinese Academy of Sciences, National Key Research and Development Program of China, NIBS and Tsinghua Institute of Multidisciplinary Biomedical Research.
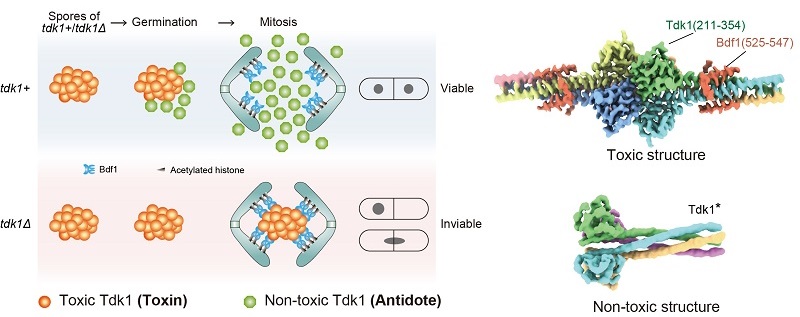
Figure: The left cartoon model illustrates how Tdk1 as both a toxin and an antidote controls mitosis of fission yeast spores. The right panel shows that the toxic and non-toxic structures of Tkd1.
(Image by YE Keqiong's group)
Article link:
https://doi.org/10.1073/pnas.2408347121
https://doi.org/10.1073/pnas.2408618121
Contact: YE Keqiong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: yekeqiong@ibp.ac.cn
(Reported by Prof. YE Keqiong's group)

