Nanozyme Targeting Hypoxic Lesions Enhances Radiosensitivity in Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma (NPC) is a malignant tumor originating from the nasopharyngeal epithelium, with radiotherapy remaining the primary treatment modality. Severe hypoxia is a critical factor contributing to increased radiotherapy resistance, tumor recurrence, and metastasis. Developing efficient and precise radiosensitization strategies is an urgent clinical need for treating solid tumors such as NPC.
Nanozymes with catalase-like activity can catalyze the decomposition of hydrogen peroxide in the tumor microenvironment to generate oxygen in situ. This approach effectively avoids issues such as rapid oxygen consumption, difficult control, or significant toxicity associated with traditional methods, making it more suitable for clinical application.
On January 21, 2025, a collaborative study from the Institute of Biophysics, Chinese Academy of Sciences, the Fifth Affiliated Hospital of Sun Yat-sen University, and Guangxi Medical University was published in Nature Communications. The research developed a targeted nanozyme delivery system by identifying specific cell surface targets in hypoxic lesions of NPC, effectively enhancing the radiosensitivity of NPC through hypoxia-targeted delivery.
The researchers first analyzed clinical samples and confirmed the significant hypoxic characteristics of NPC. Subsequently, by extensively screening thousands of proteins in a cell surface protein database, they identified transferrin receptor 1 (TfR1) as highly expressed in NPC and closely associated with hypoxia, making it a potential target for hypoxic lesions.
This study found that human heavy-chain ferritin (HFn) could effectively target hypoxic lesions in NPC by specifically binding to TfR1. Using HFn as a carrier, the researchers loaded platinum nanozymes with both catalase-like activity and radiation energy absorption properties, creating a radiosensitizer (Pt-HFn) capable of efficiently targeting hypoxic lesions in NPC.
HFn significantly improved the dispersion of platinum nanozymes and enhanced catalase-like activity through a shell-core synergistic effect. In NPC xenograft models, the Pt-HFn radiosensitizer, leveraging the targeting ability of the HFn carrier, effectively accumulated in hypoxic lesions and exhibited excellent hypoxia alleviation and radiosensitization effects.
The results showed that the therapeutic efficacy of Pt-HFn combined with radiotherapy was significantly superior to that of sodium glycididazole, a widely used clinical radiosensitizer. It demonstrated higher efficacy in both single-dose and fractionated radiotherapy models without observable adverse effects.
This study provides a novel strategy for improving the efficacy of radiotherapy in NPC.
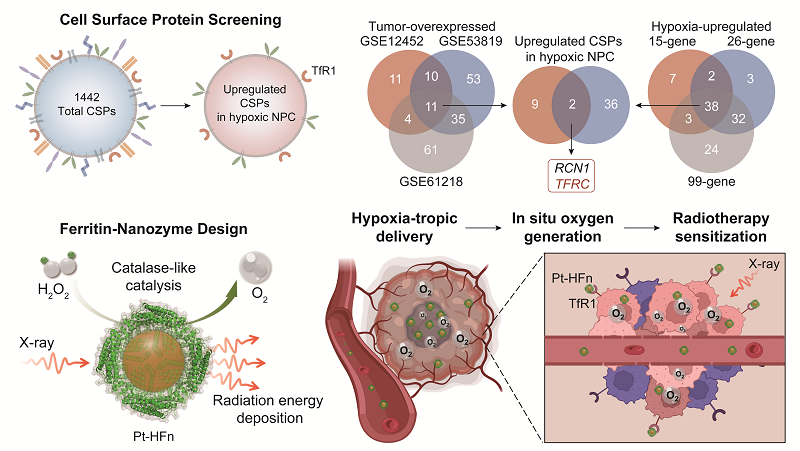
Figure. Ferritin Nanozyme Targeting TfR1 Enhances Radiosensitivity in Nasopharyngeal Carcinoma
(Image by FAN Kelong's group)
Article link: https://www.nature.com/articles/s41467-025-56134-z#Sec50
Contact: FAN Kelong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: fankelong@ibp.ac.cn
(Reported by Prof. FAN Kelong's group)

