Novel Ferritin-Based siRNA Delivery System for Targeted Glioblastoma Therapy
RNA interference (RNAi) has long been regarded as a promising approach for gene-specific therapy. Small interfering RNA (siRNA) can inhibit the expression of specific oncogenes, showing promising anti-tumor potential. However, its clinical applications have been hindered by challenges such as limited cellular uptake, insufficient lysosomal escape, poor tumor targeting and rapid renal clearance. In particular, for glioblastoma (GBM) treatment, traditional drug delivery systems encounter the significant hurdle of the blood-brain barrier (BBB). Therefore, the development of siRNA delivery carriers that can both cross the blood-brain barrier and specifically target tumors has become crucial for advancing RNAi therapies for GBM.
A recent study led by Profs. FAN Kelong and YAN Xiyun from Institute of Biophysics, CAS, published in Science Advances on February 19, 2025 , has introduced a ferritin-based siRNA delivery system targeting GBM. Their previous researches revealed that human heavy-chain ferritin (HFn) possesses specific tumor-targeting and BBB-traversing abilities. However, delivering siRNA with ferritin still faces the critical challenge of lysosomal escape. The researchers engineered ferritin to address the special requirements for siRNA delivery, successfully constructing a ferritin-based carrier with lysosomal escape functionality.
The researchers designed a series of ferritin variants with positively charged inner surfaces and truncated C-terminal. Through systematic evaluation of their structural properties and lysosomal escape capability, they obtained a novel delivery carrier-tHFn(+). This nanocarrier disassembles in the weakly acidic environment of endosomes to release siRNA while exposing internal positive charges to facilitate lysosomal escape. Cryo-electron microscopy analysis confirmed the mechanism underlying its pH responsiveness: truncation of the C-terminus weakens interfacial interactions, allowing disassembly in the acidic endosomal environment. In vitro experiments demonstrated that this carrier enables cytoplasmic delivery of siRNA, efficiently knocking down the expression of target genes. Both in vitro and in vivo studies confirmed that tHFn(+) can cross the BBB and specifically target GBM. Additionally, siTERT and siEGFR delivered by tHFn(+) exhibited remarkable therapeutic effects in mouse models.The ferritin-based carrier developed in this study demonstrated high efficiency and broad applicability in delivering siRNA molecules targeting various genes, providing a novel strategy and platform for RNAi therapy in cancer, genetic disorders, and other related diseases, with promising clinical applications.
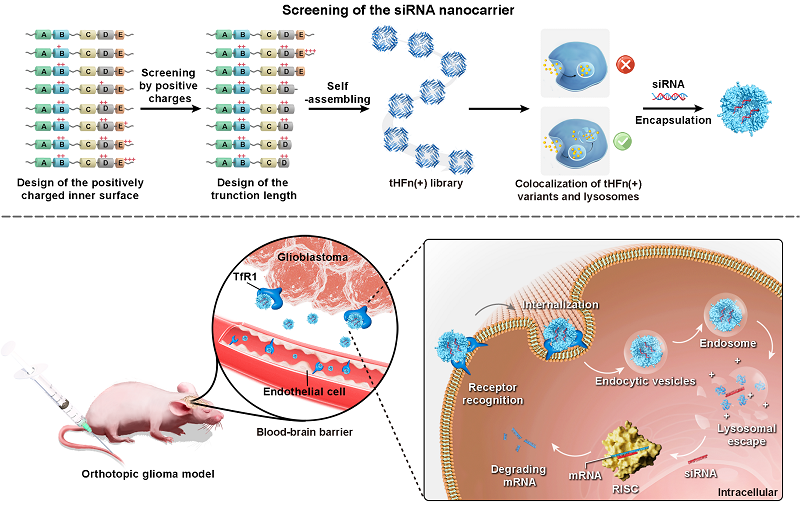
Figure. Schematic Diagram of the Design, Screening, and Application of Ferritin-based siRNA Carrier for Targeted Glioblastoma Therapy
(Image by FAN Kelong's group)
Article link: https://www.science.org/doi/10.1126/sciadv.adr9266
Contact: FAN Kelong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: fankelong@ibp.ac.cn
(Reported by Prof. FAN Kelong's group)

