Scientists Identify lncRNA as a Potential Biomarker and Therapeutic Target for HCC
Hepatocellular carcinoma (HCC) remains one of the most aggressive malignancies worldwide, ranking as the third leading cause of cancer-related deaths. In a recent study published on Nature Communications, researchers led by Profs. YANG Pengyuan and CHEN Runsheng from the Institute of Biophysics of the Chinese Academy of Sciences, have shed new light on the role of extracellular vesicles (EVs) in HCC progression, highlighting a novel mechanism by which tumor-derived long noncoding RNA (lncRNA) suppresses anti-tumor immunity.
The researchers identified HDAC2-AS2 as the most significantly differentially expressed lncRNA following TGF-β stimulation. They found that overexpression or knockdown of HDAC2-AS2 did not affect tumor cell proliferation but significantly promoted subcutaneous tumor growth in C57 mice.
Mechanistic studies revealed that tumor-derived HDAC2-AS2 could be secreted into the tumor microenvironment via EVs. Higher levels of HDAC2-AS2 were also detected in EVs from the plasma of HCC patients.
Immune function analysis showed that HDAC2-AS2 in EVs could be taken up by CD8+ T cells, where it binds to intracellular cyclin-dependent kinase 9 (CDK9), leading to a reduction in intracellular CDK9 protein levels. This, in turn, induces CD8+ T cell exhaustion and apoptosis while suppressing their cytotoxic function.
Multi-omics analysis revealed that CDK9 plays a key regulatory role in CD8+ T cell activation and cytotoxicity.
Further analysis of single-cell RNA sequencing data from HCC patients undergoing immune checkpoint blockade (ICB) therapy showed that CDK9 enhances CD8+ T cell function during ICB treatment.
Additionally, HCC tumors with high HDAC2-AS2 expression were found to benefit more from PD-1 antibody therapy.
This study demonstrates that in HBV-associated HCC, the lncRNA HDAC2-AS2, upregulated by the TGF-β signaling pathway, targets CDK9 in CD8+ T cells via EVs, inhibiting CD8+ T cell function and promoting tumor immune evasion.
These findings offer new potential biomarker and therapeutic targets for HBV- associated HCC. It provides critical insights into the molecular mechanisms of HCC immune evasion and opens new possibilities for precision medicine approaches.
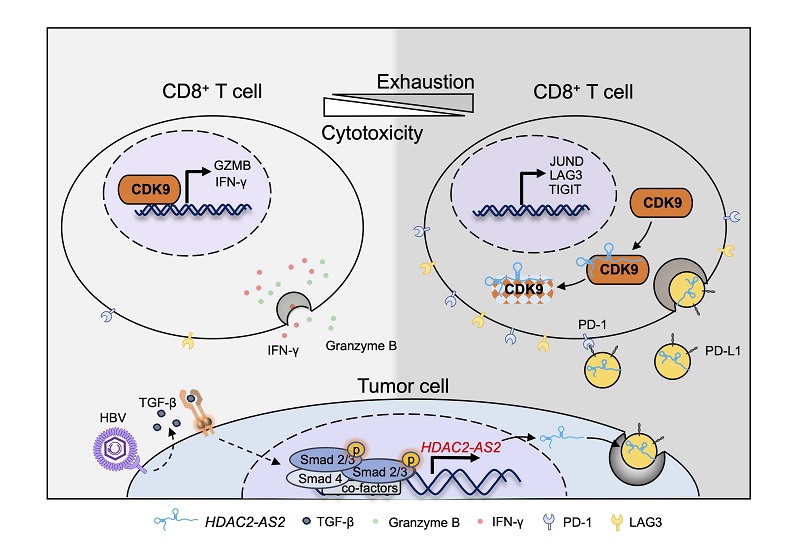
Figure. HDAC2-AS2 in tumor-derived extracellular vesicles inhibits CD8+ T cell function by targeting cytosolic CDK9
(Image by YANG Pengyuan's group)
Article link: https://www.nature.com/articles/s41467-025-57367-8
Contact: YANG Pengyuan
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: pyyang@ibp.ac.cn
(Reported by Prof. YANG Pengyuan's group)

