Scientists Reveal Ceramide mediates cell-to-cell ER stress transmission by modulating membrane fluidity
The pathological accumulation of misfolded proteins within the endoplasmic reticulum (ER) lumen induces ER stress (ERS), thereby activating the evolutionarily conserved unfolded protein response (UPR). In mammals, this adaptive signaling cascade is orchestrated through three branches: inositol-requiring enzyme 1α (IRE1α), protein kinase R-like ER kinase (PERK), and activating transcription factor 6α (ATF6α). Emerging evidence implicates UPR dysregulation as a central mechanism linking obesity - the metabolic disease nexus - to multi-organ pathology. Clinically, UPR activation is observed in adipose tissue of obese individuals and hepatocytes during Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) progression. These observations suggest potential inter-tissue crosstalk through UPR signaling, which may drive systemic metabolic deterioration.
In a recent publication in Journal of Cell Biology, 'Ceramide mediates cell-to-cell ER stress transmission by modulating membrane fluidity' (DOI: 10.1083/jcb.202405060), Dr. HUO Yazhen et. al. from the laboratory of Dr. WANG Likun in the Institute of Biophysics, Chinese Academy of Sciences, demonstrated that stressed adipocytes transmit ER stress signals to hepatocytes via ceramide, a lipid mediator modulating membrane fluidity.
The authors observed that ER-stressed adipocytes secrete bioactive factors that broadly activate UPR signaling in hepatocytes. Lipidomics profiling identified ceramide as a critical factor enriched in conditioned media from stressed adipocytes. Ceramide depletion abolished UPR activation in hepatocytes, confirming its essential role. Intriguingly, this ceramide secretion was regulated through an UPR-independent mechanism involving adipocyte-derived acid sphingomyelinase (ASM), which catalyzes extracellular sphingomyelin hydrolysis to generate ceramide. Addressing ceramide's inherent hydrophobicity, the study systematically excluded small extracellular vesicle (sEV)-mediated transport, instead identifying high-density lipoprotein (HDL) as the principal ceramide carrier.
In recipient hepatocytes, ceramide incorporation induced biophysical remodeling of plasma and ER membranes, reducing membrane fluidity. This physicochemical alteration impaired sarco/endoplasmic reticulum Ca²⁺-ATPase (SERCA) function, causing ER calcium depletion and subsequent UPR activation. Notably, sphingomyelin supplementation restored membrane fluidity and abrogated UPR signaling.
The research team further established the pathophysiological relevance of this mechanism across multiple cellular contexts: cancer cell-macrophage and hepatocyte-hepatocytes exhibited analogous ceramide-mediated ERS signal transmission, universally reversible by sphingomyelin intervention. These findings suggest a conserved intercellular stress signaling paradigm with broad implications for chronic disease pathogenesis.
This study elucidates a paradigm-shifting mechanism: ceramide serves as a lipid second messenger mediating intercellular ERS propagation via membrane biophysical modulation. The discovery not only redefines our understanding of metabolic disease systematically but also establishes lipid-targeted therapeutics as a novel frontier for combating obesity-related pathologies.
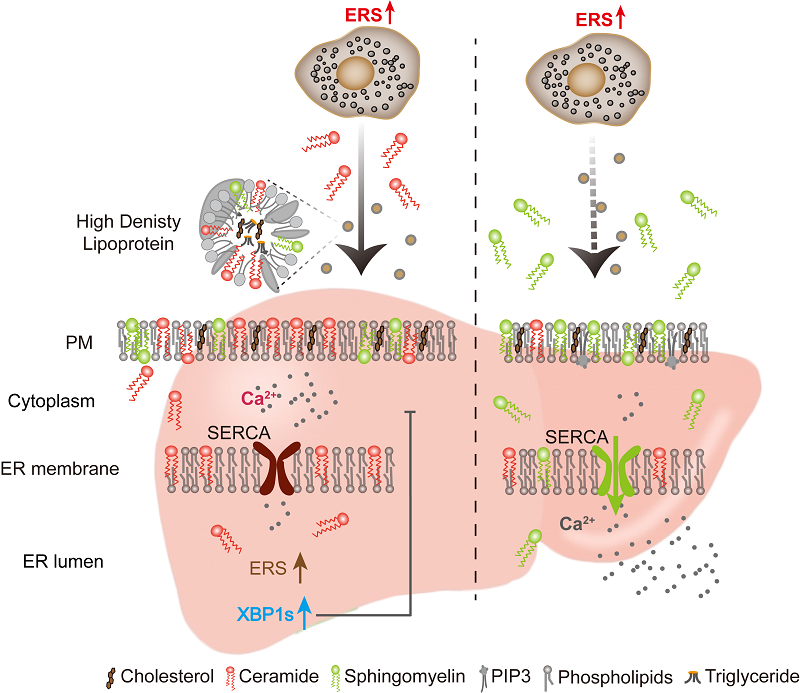
Proposed model showing adipocytes underling ERS secrete ASM to generate ceramide which is transported via HDL. This process reduces membrane fluidity, impairs SERCA activity, disrupts calcium homeostasis, and thereby activates the UPR.
(Image by HUO Yazhen)
Article link: https://doi.org/10.1083/jcb.202405060
Contact: WANG Likun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Tel: 86-010-64881162
E-mail: wanglikun@ibp.ac.cn
(Reported by Prof. WANG Likun 's group)

