Isocyanic acid is an anti-inflammatory metabolite that limits the NLRP3 inflammasome activation
Numerous studies have shown that macrophages may undergo metabolic reprogramming in response to pathogen invasion. Laccase domain containing 1 (LACC1), also known as C13orf31 or FAMIN, is a metabolic enzyme highly expressed in inflammatory macrophages and serves as a central regulator for immune-metabolic function in classically activated macrophages. LACC1 may act as an isocyanic acid (ICA) synthase that catalyzes the cleavage of citrulline into ornithine and ICA. Exposure of proteins to ICA results in carbamoylation, a post-translational modification resulting from the non-enzymatic reaction of ICA with ε-amino group of lysine residue. It has been known that ICA-mediated protein carbamoylation is associated with the development of renal and cardiovascular disease. However, it remains unknown whether ICA serve as immune effectors.
Inflammasomes are activated in response to microbial invasion and damage signals. Numerous different types of inflammasomes and their corresponding activating stimuli have been identified. Among them, the NLRP3 inflammasome has been studied extensively and is activated by a wide spectrum of stimuli. Activation of the NLRP3 inflammasome triggers autocatalytic activation of caspase-1 (CASP1), leading to processing the proinflammatory cytokines interleukin-1β (IL-1β) and interleukin-18 (IL-18) for their maturation, and cleaving gasdermin D (GSDMD) to induce pyroptosis. It is known that aberrant activation of NLRP3 inflammasome is the etiology of a series of inflammatory, autoimmune, and degenerative diseases, thus a better understanding of the molecular mechanisms underlying NLRP3 inflammasome activation remains necessary.
Recently, Dr. Li, Xinjian's group from the Institute of Biophysics, Chinese Academy of Sciences, published a research paper entitled "Isocyanic acid-mediated NLRP3 carbamoylation reduces NLRP3-NEK7 interaction and limits inflammasome activation" in the 《Science Advances》journal.
This work reveals that ICA is a de novo anti-inflammatory metabolite that limits the NLRP3 inflammasome activation (Figure 1). ICA directly carbamoylates NLRP3 at lysine 593 to disrupt NLRP3-NEK7 interaction, a key step in assembly of active NLRP3 inflammasome. Abrogation of ICA biosynthesis by LACC1/Lacc1 knockout or expression of K593 carbamoylation (K593ca)-deficient NLRP3 mutant promotes macrophagic inflammatory response in vitro. Furthermore, Lacc1-/- mice and mice harboring K593ca-deficient NLRP3 mutation manifest exacerbated inflammatory response in vivo. Hence, these findings identify ICA as an endogenous immunoregulatory metabolite that limits NLRP3-driven inflammation and provide valuable insights into the regulation of NLRP3 inflammasome activation, governed by metabolites.
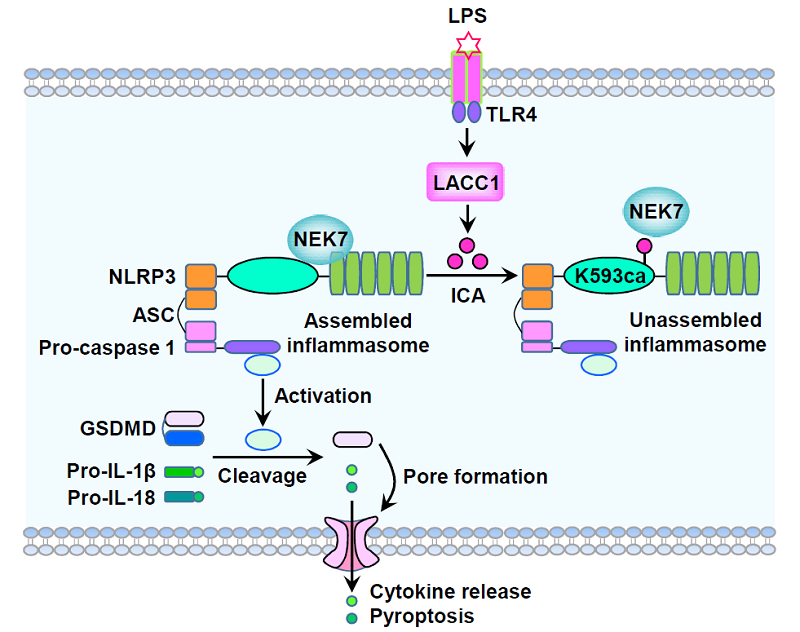
Figure 1. A schematic diagram shows isocyanic acid (ICA)-mediated NLRP3 K593ca limits NLRP3-driven inflammation. ICA, a metabolite synthesized by LACC1 in lipopolysaccharide (LPS)-stimulated macrophages, directly carbamoylates NLRP3 at K593. ICA-mediated NLRP3 K593 carbamoylation (K593ca) suppresses NLRP3 inflammasome assembly by disrupting the interaction between NLRP3 and NEK7, thereby limiting pyroptosis triggered by NLRP3 inflammasome activation.
(Image by LI Xinjian 's group)
Article link: https://www.science.org/doi/10.1126/sciadv.adq4266
Contact: LI Xinjian
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: lixinjian@ibp.ac.cn
(Reported by Prof. LI Xinjian 's group)

