Scientists Unravel Molecular Mechanism of ARF Proteins in Vesicle Fission
The formation of intracellular transport vesicles is a critical cellular function that ensures the correct localization of proteins and lipids within the cell. This process involves recruitment of coat proteins to compartmental membranes to initiate vesicle budding, followed by vesicle fission to release fully formed transport vesicles. While significant progress has been made in understanding the role of ADP-Ribosylation Factor (ARF) small GTPases in coat recruitment and vesicle budding, their precise function in vesicle fission has remained unclear.
In a study published in PNAS on March 21, 2025, Prof. SUN Fei's team from the Institute of Biophysics of the Chinese Academy of Sciences, along with collaborators from Pohang University of Science and Technology, Harvard Medical School, and City University of Hong Kong, revealed how ARF proteins contribute to vesicle fission by directly tubulating membranes.
The researchers utilized cryoelectron microscopy and helical reconstruction techniques to successfully capture the unique helical lattice structure formed by ARF6 protein on the lipid membrane surface. The ARF6 tetramer serves as the fundamental assembly unit, stabilized primarily by hydrophobic interactions and electrostatic interactions within the tetramer, while inter-tetramer interactions rely on electrostatic forces to form a stable helical structure. Molecular dynamics simulations further validated the assembly stability of the ARF6 tetramer and revealed that its stable association with the lipid membrane is achieved through the synergistic action of the N-terminal myristoyl chain (MYR) and amphipathic helices (AH).
The study also discovered that mutations targeting the ARF6 lattice interaction interfaces and the N-terminal AH specifically blocked its function in mediating endocytic recycling transport. This finding confirmed the critical roles of these interaction interfaces and amphipathic helices in the endocytic recycling process. Functional experiments demonstrated that GTP hydrolysis of ARF6 triggers neck scission, leading to the formation of small vesicles and completing the vesicle fission process.
Given the high homology between ARF1 and ARF6, the researchers conducted COPI complex vesicle reconstitution experiments, demonstrating that the assembly mechanism of ARF is the core driver of vesicle fission. The study confirmed that mutations targeting the ARF lattice interaction interfaces significantly impaired vesicle fission, corroborating prior research that ARF1 dimerization is essential for vesicle fission and that the dimer interface precisely aligns with the tetramer interface region in the helical lattice.
This work not only elucidates the detailed molecular mechanism of ARF proteins in vesicle fission, filling a critical knowledge gap in the final stage of vesicle formation, but also provides new insights for future research on the regulation of intracellular material transport.
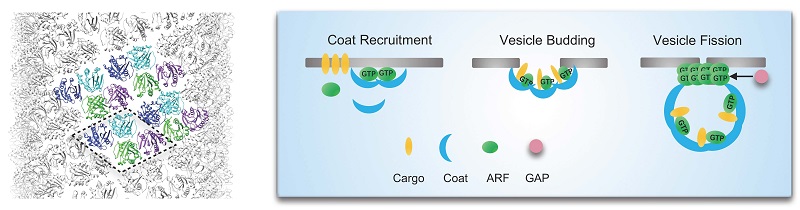
Figure. Molecular Mechanism of ARF6-Mediated Vesicle Fission: The left side shows the helical lattice structure of membrane-bound ARF6, while the right side illustrates the vesicle formation process.
(Image by SUN Fei's group)
Article link: https://www.pnas.org/doi/10.1073/pnas.2417820122
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: feisun@ibp.ac.cn
(Reported by Prof. SUN Fei's group)

