Scientists uncovered a novel function of autophagy protein ATG-9 in regulating lysosome integrity
Recently, a research team led by Prof. ZHANG Hong from the Institute of Biophysics, Chinese Academy of Sciences published an article in Journal of Cell Biology on April 9, 2025, titled "The autophagy protein ATG-9 regulates lysosome function and integrity". They have, for the first time, revealed the molecular mechanism by which the key autophagy protein ATG-9 regulates phospholipid scramblase activity to facilitate the repair of damaged lysosomes. This discovery provides a new research direction for therapeutic strategies targeting lysosomal dysfunction-related diseases.
ATG-9 is a protein localized on 30-60 nm vesicles and plays multiple roles in autophagosome formation. For example, in yeast cells, it acts as a "membrane seed" to recruit autophagy machinery and promote the formation of autophagic isolation membranes. Additionally, ATG-9 possesses phospholipid scramblase activity, which regulates the asymmetric distribution of membrane lipids, influencing membrane morphology and function, thereby facilitating the extension of autophagic isolation membranes. Notably, ATG-9 also exhibits functions independent of autophagy. In mammalian cells, ATG9A can be recruited to sites of plasma membrane damage, where it orchestrates the ESCRT machinery to repair membrane lesions.
Dr. Zhang's team discovered that in Caenorhabditis elegans epg-5 mutants, ATG-9 vesicles selectively accumulate around mildly damaged lysosomes, and this process is independent of the autophagy marker LGG-1 (the worm homolog of LC3). Surprisingly, ATG-9 mutants with reduced phospholipid scramblase activity significantly enhance the repair of mildly damaged lysosomes, restore lysosomal activity, and rescue autophagy defects in epg-5 mutants.
Further investigation demonstrated that reducing phosphatidylethanolamine (PE) synthesis results in a phenotype similar to the inhibition of ATG-9 scramblase activity. Specifically, decreased PE synthesis also promotes lysosomal repair and rescues autophagy defects in epg-5 mutants. These findings suggest that ATG-9 mutants with attenuated phospholipid scramblase activity may directly or indirectly alter the phospholipid distribution between the inner and outer leaflets of lysosomal membranes, thereby inducing membrane curvature to facilitate lysosome biogenesis and repair.
Lysosomal dysfunction and loss of lysosomal integrity are closely associated with lysosomal storage diseases and neurodegenerative disorders. The phospholipid scramblase activity of ATG-9 identified in this study provides new insights into the pathogenesis of lysosomal dysfunction-related diseases and represents a potential druggable target for therapeutic intervention.
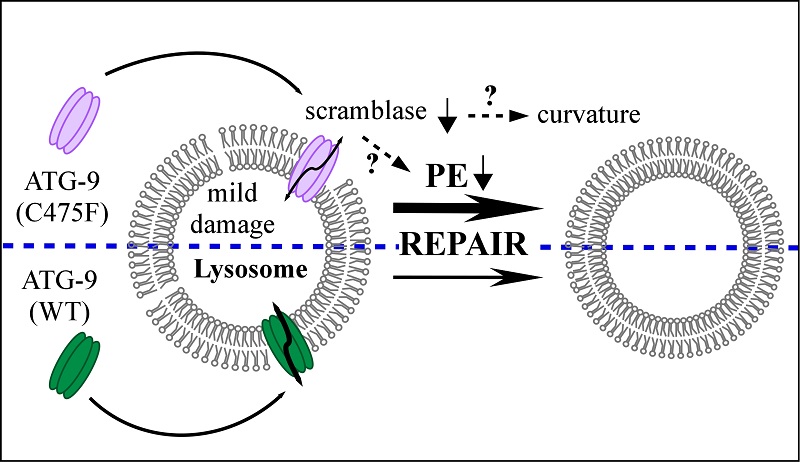
Fig. Model for the involvement of ATG-9 in repair of damaged lysosomes
(Image by ZHANG Hong's group)
Article link: https://rupress.org/jcb/article/224/6/e202411092/277375/The-autophagy-protein-ATG-9-regulates-lysosome
Contact: ZHANG Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: hongzhang@ibp.ac.cn
(Reported by Prof. ZHANG Hong's group)

