Scientists found transcription factor LIN-15B negatively regulates autophagy and lysosome function in C. elegans
Key steps of the autophagy pathway include autophagosome formation, autophagosome-lysosome fusion, and lysosomal degradation. Transcriptional regulation of genes involved in the autophagy-lysosome pathway is an important mechanism for controlling autophagic flux. In mammalian cells, TFEB (transcription factor EB) acts as a master transcription factor to control the expression level of genes functioning in autophagosome formation and maturation. The C. elegans TFEB homolog HLH-30 is also involved in autophagy regulation in response to stress and aging. However, loss of function of hlh-30 causes no evident autophagy defect during development. It remains unknown whether autophagy is modulated through regulation of global transcription during C. elegans development.
On April 2, 2025, a research team led by Prof. ZHANG Hong from the Institute of Biophysics, Chinese Academy of Sciences published an article in Autophagy, titled " T16G12.6/IMPORTIN 13-mediated cytoplasm-to-nucleus transport of the THAP transcription factor LIN-15B controls autophagy and lysosome function in C. elegans". In this article, they showed that the THAP domain-containing transcription factor LIN-15B modulates autophagy activity during C. elegans development.
lin-15B is a SynMuv B gene that functions in specification of vulva cell fate, cell cycle progression, larval growth, gonadogenesis, and pharyngeal morphogenesis in C. elegans. Simultaneous loss of function of SynMuv B genes with either SynMuv A or SynMuv C genes results in a multivulva phenotype. However, loss of function of other SynMuv genes cannot suppress the phenotype of autophagy mutants, suggesting that lin-15B has a specific role in autophagy regulation distinct from other SynMuv genes.
Scientists found that loss of function of lin-15B suppresses the autophagy defect caused by impaired autophagosome maturation and promotes lysosome biogenesis and function. The cytoplasm-to-nucleus translocation of LIN-15B is mediated by the T16G12.6/IMPORTIN 13/IPO-13 receptor and modulated by nutrient status. In control animals, LIN-15B mainly localizes in the nucleus to repress the expression of target genes. The transcription of autophagy genes occurs at a basal level. In ipo-13 mutant animals or starved animals, the nuclear level of LIN-15B is reduced. The expression of autophagy-lysosome pathway genes is upregulated and autophagy flux is enhanced.
In summary, this study reveals that LIN-15B plays a critical role in regulating autophagy activity during C. elegans development,and that its regulatory function relies on the nuclear import protein IPO-13 to transport it from the cytoplasm into the nucleus, a process that is modulated by the cellular nutrient conditions.
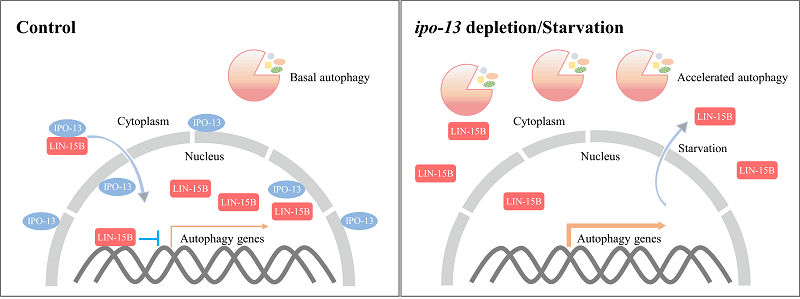
Figure. Model showing that IPO-13-mediated cytoplasm-to-nucleus transport of LIN-15B controls autophagy activity.
(Image by ZHANG Hong's group)
Article link:https://www.tandfonline.com/doi/full/10.1080/15548627.2025.2482724
Contact: ZHANG Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: hongzhang@ibp.ac.cn
(Reported by Prof. ZHANG Hong's group)

