Beyond On and Off: Researchers Detail How Cells "Prepare" Their Genes for the Future
In multicellular organisms, although nearly all cells possess the exact same DNA, they exhibit markedly different responses to the same signals. Past exposure to signals can even influence a cell's future response patterns. The key to explaining these biological phenomena lies in epigenetics. While traditional views hold that the primary function of epigenetics is to maintain the on or off state of genes, recent cutting-edge findings reveal that cells not only maintain existing homeostasis but also actively prepare for future gene expression. This preparation does not involve immediately activating genes, but rather establishing a dynamic epigenetic barrier to precisely regulate the speed and intensity of a gene's response to future signals. By regulating chromatin structure, DNA methylation, and histone modifications, cells can establish a tunable response state for specific genes. This state can be stably inherited by daughter cells while retaining the flexibility to respond to future signals, creating a sophisticated balance between genomic stability and plasticity.
On June 16, 2025, Dr. ZHU, Bing and Dr. XIONG Jun from the Institute of Biophysics, Chinese Academy of Sciences, published a review article titled "Epigenetic Preparation of Future Gene Induction Kinetics" online in the journal Annual Review of Genetics.
The article systematically elaborates on how cells use epigenetic mechanisms to preset gene expression states, thereby influencing the activation efficiency and kinetics of future gene induction. The review summarizes four core epigenetic preparation mechanisms: (1) Priming, where cells lower the activation threshold of specific genes in advance, putting them in a poised state for rapid and efficient activation upon receiving developmental or environmental signals; (2) Reining, the opposite of priming, where cells maintain a relatively high barrier for activation to keep genes in a state of "restrained and delayed activation", preventing premature or excessive expression; (3) Transcriptional memory, where cells remember past gene activation events, allowing genes with this memory to respond with greater speed and strength upon re-encountering the same or similar stimuli; (4) Transcriptional tolerance, where an initial strong signal raises the gene's activation barrier, leading to a weakened or even absence of response to subsequent stimuli. This negative feedback is crucial for preventing tissue damage from excessive inflammatory responses.
The review further untangles the molecular mechanisms behind these preparatory processes and proposes a working model for epigenetic preparation. It engages in an in-depth discussion of key questions regarding gene selectivity, the mechanisms for the stable inheritance of prepared chromatin states across cell divisions, and the key strategies for the dynamic regulation of the epigenetic barrier by cells. It also provides new insights for future research directions and for intervening in developmental abnormalities, autoimmune diseases, and cancer.
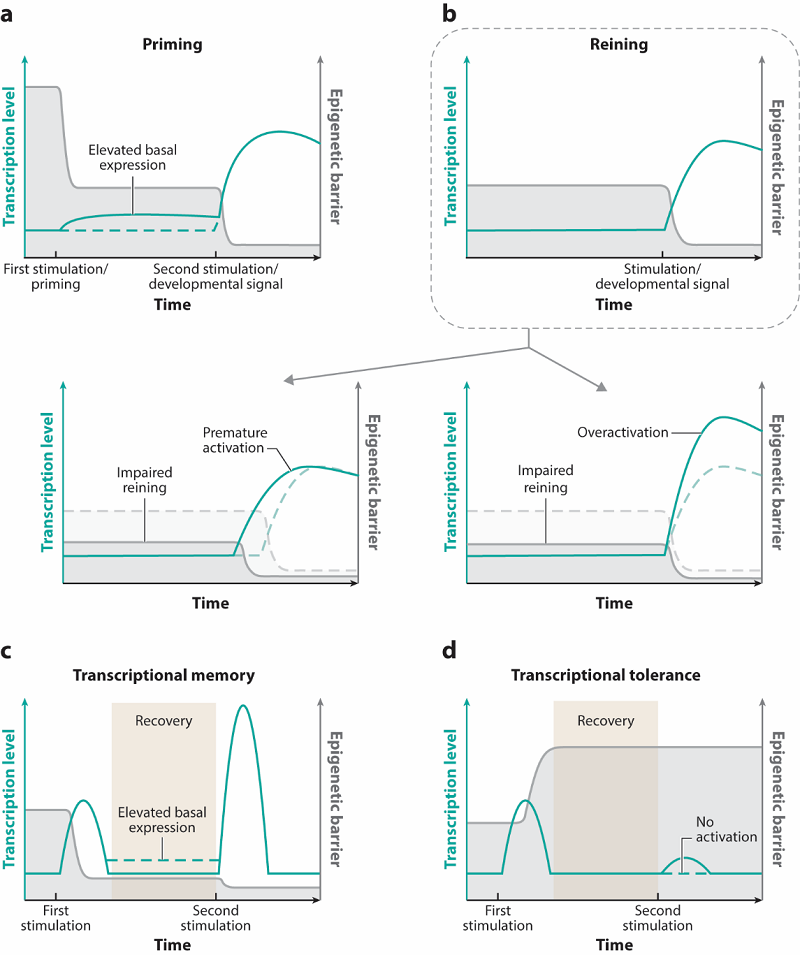
Figure 1. Major processes of epigenetic preparation for future gene induction
(Image by ZHU Bing's group)
Article link: https://doi.org/10.1146/annurev-genet-012825-093148
Contact: ZHU Bing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: zhubing@ibp.ac.cn
(Reported by Prof. ZHU Bing's group)

