Spatial Proximity Between LAG-3 and TCR Suppresses T Cell Activation, Revealing New Therapeutic Strategy for Autoimmune Diseases
Immune checkpoint pathways regulate T cell function and play pivotal roles in treating both cancer and autoimmune diseases. Among these, LAG-3 (Lymphocyte Activation Gene 3)-a classical immunosuppressive receptor-has long presented unresolved mysteries regarding its biological mechanisms.
On June 30, 2025, a collaborative study by Prof. WANG Jun's team from NYU Grossman School of Medicine, Prof. LOU Jizhong's group from the Institute of Biophysics of Chinese Academy of Sciences, and Prof. CHEN Wei's team from Zhejiang University School of Medicine was published online in Cell. The study, titled "Proximity between LAG-3 and the T Cell Receptor Guides Suppression of T Cell Activation and Autoimmunity", systematically reveals that LAG-3 inhibits T cells through spatial proximity to the TCR (T Cell Receptor) complex rather than simple ligand binding. Leveraging this discovery, the team innovatively developed a LAG-3/TCR Bispecific T cell Silencer (BiTS), demonstrating potent efficacy across multiple autoimmune disease models.
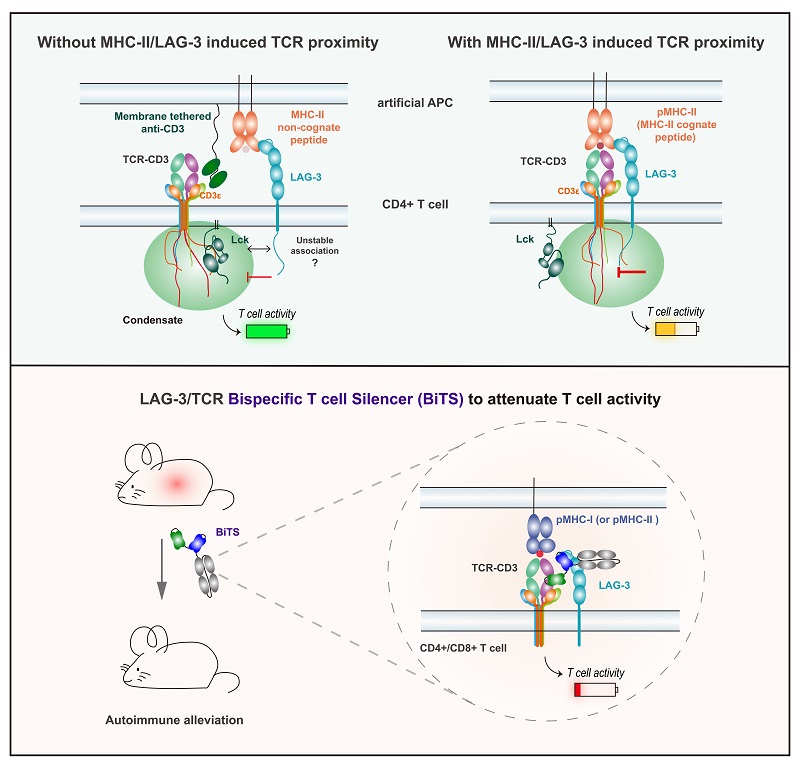
The study revealed several key findings. First, using engineered artificial antigen-presenting cells (aAPCs), the authors found that MHC-II (Major Histocompatibility Complex class II) ligand binding alone is insufficient to trigger LAG-3 mediated suppression in CD4+ T cells. Effective inhibition occurs only when MHC-II simultaneously engages both LAG-3 and TCR to form a ternary complex. This "cis-proximity" mechanism redefines the classical ligand-receptor model of LAG-3 and explains its weak functionality in CD8+ T cells. Second, investigating the molecular basis of this cis-proximity, the team discovered that LAG-3's intracellular domain forms liquid-liquid phase-separated condensates with CD3ε. These condensates competitively disrupt CD3ε-Lck kinase interactions, ultimately attenuating TCR signal transduction. Third, capitalizing on this mechanism, the researchers designed BiTS-an antibody bridging LAG-3 and TCR. BiTS artificially enforces LAG-3-TCR proximity, enabling potent suppression of TCR signaling and T cell activation. Unlike conventional PD-1 agonists, this inhibition is independent of CD4 co-receptors.
BiTS exhibited robust efficacy in diverse autoimmune models, including type 1 diabetes,experimental autoimmune encephalomyelitis (EAE; a multiple sclerosis model), and T cell mediated hepatitis (simulating pediatric hepatitis of unknown etiology). Treatment significantly suppressed pathogenic T cell responses and ameliorated tissue damage.
The study proposes "proximity-dependent immune checkpoints" as a new paradigm, demonstrating that LAG-3 suppression relies on spatial co-localization with the TCR rather than canonical ligand binding. This conditional mechanism clarifies LAG-3's limited efficacy as a monotherapy in cancer while supporting its "repositioning" for autoimmune diseases. Furthermore, the "cis-inhibitory bispecific antibody" strategy pioneers a novel therapeutic approach to precisely target pathogenic T cells, opening new avenues for treating autoimmune disorders.
Artice link: https://www.cell.com/cell/fulltext/S0092-8674(25)00638-5
Contact: LOU Jizhong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: jlou@ibp.ac.cn
(Reported by Prof. LOU Jizhong's group)

