M Protein Topology Holds the Key to SARS-CoV-2 Assembly and Replication
SARS-CoV-2, the virus responsible for COVID-19, encodes four structural proteins: spike (S), envelope (E), nucleocapsid (N), and membrane (M). Among them, the M protein is the most abundant and plays a central role in orchestrating viral assembly. While the membrane topologies of S and E have been characterized, the configuration of M remained unclear-until now.
In a study published in Science Bulletin on June 16, 2025, a team led by Prof. HU Junjie from the Institute of Biophysics of Chinese Academy of Sciences has uncovered the unexpected membrane topology of the SARS-CoV-2 M protein and identified its critical role in viral assembly and replication.
The research provides unprecedented detail about how M coordinates with other structural proteins and navigates the host cell's internal membrane system to enable the formation of infectious viral particles.
The researchers found that both the N- and C-termini of the SARS-CoV-2 M protein face the cytoplasmic side. Further analysis demonstrated that the first "transmembrane domain" is not fully integrated into the membrane, resulting in a non-canonical topology that prevents glycosylation at the N-terminal site. This unusual feature appears to be conserved among other betacoronaviruses.
The proper orientation of the M protein was found to be highly sensitive to the composition of its N-terminal amino acids, and the addition of purification tags at the N-terminus significantly increased the likelihood of misorientation. Therefore, caution should be exercised when interpreting membrane orientation based on purified protein structures, as they may not accurately reflect native conditions.
During viral assembly, the correct topology of the M protein is essential for recruiting the S and E structural proteins. Failure of the M protein to localize properly or to recruit other structural components effectively impairs virus assembly. This mechanism of assembly is conserved among betacoronaviruses.
The study suggests that disrupting the topology and membrane trafficking of the M protein could serve as a broad-spectrum strategy to inhibit coronavirus assembly.
These findings offer valuable insight into coronavirus biology and open new avenues for antiviral strategies targeting viral assembly.
"Understanding the true topology and trafficking signals of the M protein is fundamental." said Prof. HU. "It's a missing puzzle piece in how SARS-CoV-2 builds infectious virions-and one that could inform future therapeutic development."
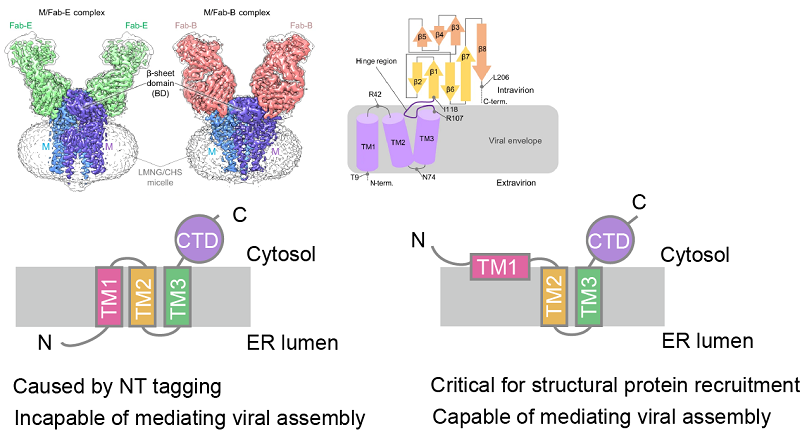
Figure 1. Membrane topology of the M protein
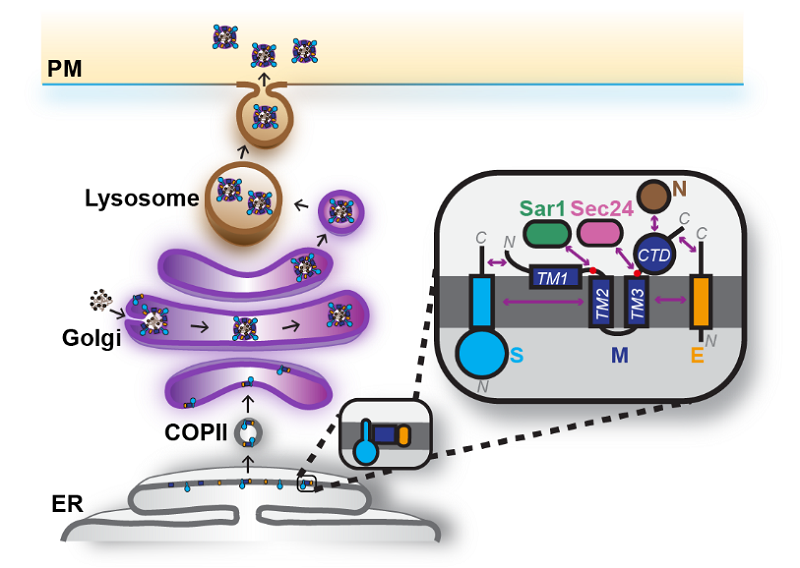
Figure 2. Working model of coronavirus assembly
(Image by HU Junjie's group)
Article link: https://doi.org/10.1016/j.scib.2025.06.012
Contact: HU Junjie
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: huj@ibp.ac.cn
(Reported by Prof. HU Junjie's group)

