ZHU Ping's Group and Collaborators Reveal the Genome Architecture and Infection Strategy of a Novel Archaeal Enveloped Virus
The three-domain system classifies all life on earth into Bacteria, Archaea, and Eukarya. Among these, Archaea are widely distributed in extreme environments such as high temperatures, strong acidity or alkalinity, and hypersaline conditions, and are considered key subjects for studying the origins of life and survival mechanisms under extreme conditions. Viruses that infect Archaea, known as archaeal viruses, exhibit remarkable morphological diversity-including spindle-shaped, bottle-shaped, droplet-shaped, filamentous, and rod-shaped forms. The discovery of these diverse forms has sparked significant interest in understanding how archaeal viruses adapt to extreme environments.
Previous research on archaeal viruses has primarily focused on morphologically uniform filamentous, spindle-shaped, and icosahedral viruses, which typically rely on highly compact organizational structures to withstand harsh environmental stresses. For example, filamentous viruses package their genomic DNA in the A-form through their major capsid proteins and employ extensive hydrophobic interactions among proteins to shield the genome from extreme pH conditions. In contrast, archaeal enveloped viruses are characterized by higher structural flexibility, lower uniformity, and a lack of symmetry, making them challenging targets for structural determination by cryo-electron microscopy (cryo-EM) single-particle 3D reconstruction methods. Moreover, infection strategies of archaeal viruses under extreme conditions remain poorly understood, with limited in situ evidence and insufficient resolution to elucidate these processes.
Therefore, in-depth investigations into the structural characteristics of archaeal enveloped viruses and their adaptation mechanisms in extreme environments are essential to fill this knowledge gap. Such studies will provide novel insights into virus-host interactions under extreme conditions and advance our understanding of archaeal virology.
On July 18, 2025, Professor ZHU Ping's research group at the Institute of Biophysics, Chinese Academy of Sciences, in collaboration with Professor HUANG Li's group at the Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou)/Institute of Microbiology, Chinese Academy of Sciences, and Dr. WANG Haina from China University of Geosciences (Beijing), published a research article entitled "Insights into the spool-like architecture and infection strategy of an enveloped archaeal virus" in Science Advances. In this study, the researchers performed cryo-electron microscopy (cryo-EM) structural analysis of the unique nucleocapsid architecture of Sulfolobus ellipsoid virus 1 (SEV1), an archaeal enveloped virus isolated from a highly acidic hot spring in Costa Rica (86-106°C, pH 2.2-2.5). They further explored its infection strategy for the first time using cryo-focused ion beam (cryo-FIB) milling combined with cryo-electron tomography (cryo-ET), shedding new light on the structural diversity, adaptation mechanisms, and life cycle of archaeal viruses in extreme environments.
The research team first enriched virus particles and collected cryo-ET datasets. Structural analysis revealed that SEV1's nucleocapsid consists of nucleoprotein filaments formed by the binding of its genome DNA with the major nucleoprotein. These nucleoprotein filaments coil multiple times in-plane along the longitudinal axis of the virion, forming disc-like structures reminiscent of mosquito coils. Stacking of multiple such discs creates a compact helical spool-like architecture. Moreover, the nucleoproteins are arranged in a "beads-on-a-string" pattern along the genome, exhibiting a degree of periodicity. Using a technology named sub-tomogram averaging (STA), the researchers further analyzed different regions of the SEV1 virion. The averaged results showed that the nucleoproteins are arranged in a lattice-like pattern within the nucleocapsid, maintaining relatively uniform inter-protein distances. The genome is packaged by the nucleoproteins into a mosquito coil-like configuration, which forms a central channel through the stacked coil layers. STA provided precise structural parameters for these features.
Biochemical analyses identified VP4 as the major capsid protein of SEV1, corresponding to the aforementioned "beads-on-a-string" nucleoprotein. Combined with AlphaFold-based structural prediction and protein-DNA interaction modeling, the results indicate that VP4 forms a homodimer, with each monomer comprising five α-helices. The dimerization creates a central channel through which the DNA passes. This architecture is consistent with previously reported models of protein-DNA interactions in filamentous and icosahedral archaeal viruses. Although VP4 has very few homologous sequences in public databases, phylogenetic analyses of the retrieved homologs revealed highly conserved tertiary structures despite low sequence identity. Notably, these homologs are all derived from extreme thermoacidophilic environments in the Pacific region, including hot springs in Yellowstone National Park (USA), Japan, and deep-sea sediments in the Gulf of California, Mexico. Based on these observations, the researchers propose that the formation of α-helical nucleoprotein dimers to encapsulate the genome may represent a conserved strategy by which archaeal viruses protect their genetic material in extreme environments. Integrating structural biology and bioinformatics, they proposed a novel packaging model for SEV1, termed the "coil-stacking" model.
In addition to typical virions that conform to the coil-stacking model, the researchers also observed atypical virions both within infected host cells and among purified particles. These atypical particles exhibited partial inversion of their nucleocapsid, with the mosquito coil-like structure flipped in certain regions, indicating structural flexibility in the SEV1 nucleocapsid. Although the overall coil-stacking arrangement remains, the nucleoprotein filaments appear highly flexible. To investigate how such structural flexibility adapts to extreme conditions, the researchers subjected intact virions and isolated nucleocapsids to simulated extreme pH (0.5-13) and temperature (25-100°C) conditions. Remarkably, while the intact virions maintained their structural integrity at high temperatures, the nucleocapsids were completely disrupted once the envelope was removed, highlighting the critical role of the envelope in stabilizing the genome-a strategy distinct from the rigid architectures typical of filamentous archaeal viruses reported previously.
Furthermore, the team used cryo-FIB and cryo-ET to visualize the SEV1 infection process in its archaeal host cells. Segmentation analyses revealed that in healthy host cells, the cytoplasm is evenly distributed and the S-layer is closely attached to the cell membrane. Upon SEV1 infection, the host cytoplasm undergoes significant reorganization, condensing into patch-like regions and forming large, dense "viral factories." Within these regions, the researchers captured a continuum of viral assembly intermediates, including nucleoprotein filaments being coiled and assembled into mature progeny virions encapsulated within spike envelopes, all exhibiting the characteristic coil-stacking nucleocapsid. These observations demonstrate that both assembly and envelope acquisition occur entirely within the host cytoplasm, indicating that, unlike many other known enveloped viruses, SEV1 acquires its envelope intracellularly rather than budding from the host cell membrane. Mature SEV1 virions are then released through characteristic hexagonal pyramid structures known as virus-associated pyramids (VAPs) that open at the host cell surface. At the late stage of infection, the host cell is nearly emptied as cellular contents are exhausted.
These findings highlight VAPs as key structures for SEV1 release. To determine the protein composition of VAPs, the researchers performed secondary structure prediction and functional validation of viral genes, confirming that SEV1-encoded ORF84 is likely responsible for forming VAPs. When heterologously expressed in the genetically tractable Sulfolobus islandicus E233S system, ORF84 could induce similar multi-lobed openings. Interestingly, whereas SEV1-ORF84 forms hexagonal pyramids in its native host Sulfolobus sp. A20, it forms heptagonal structures in S. islandicus E233S, suggesting that host factors also contribute to VAP morphogenesis.
Together, these discoveries provide a detailed depiction of SEV1's intracellular assembly, envelope acquisition, and release process, representing the first application of cryo-FIB to study the life cycle of an archaeal virus. The results reveal a unique assembly and release strategy evolved by archaeal viruses to survive and propagate in extreme environments.
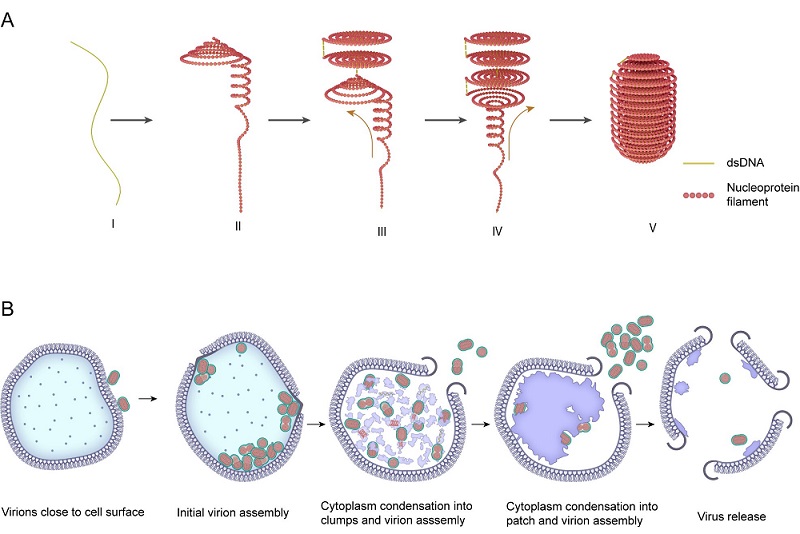
Fig. 1 The genomic assembly and infection process of SEV1
(Image by ZHU Ping's group)
Article link: https://www.science.org/doi/10.1126/sciadv.adv7326
Contact: ZHU Ping
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: zhup@ibp.ac.cn
(Reported by Prof. ZHU Ping's group)

