hG6PC1 Cryo-EM Structures Reveal Catalytic Mechanism and Guide Glucose Metabolism Therapy
On July 15, 2025, a new study, published in Cell Discovery, led by Prof. ZHAO Yan from the institute of Biophysics of the Chinese Academy of Sciences has unveiled high-resolution cryo-electron microscopy (cryo-EM) structures of the human glucose-6-phosphatase catalytic subunit 1 (hG6PC1), providing new insights into its function in energy metabolism and its role in glucose metabolic genetic disorders.
HG6PC1 is a key enzyme in glucose metabolism that regulates the final common step of gluconeogenesis and glycogenolysis, directly influencing energy homeostasis. Dysfunction or mutations of hG6PC1 can lead to a severe metabolic genetic disorder known as glycogen storage disease type 1a (GSD-1a). Moreover, elevated hG6PC1 activity is closely associated with disrupted glucose metabolism in diabetes, making it a critical therapeutic target for treating glucose metabolism disorders.
In this study, the researchers reported the open conformation of hG6PC1 in its ligand-free (apo) state, along with partially open conformations when bound to the substrates glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P), as well as an open complex structure bound to the product phosphate.
Despite structural differences between the formula of G6P and F6P, both substrates bind within a similar pocket of G6PC1. Key residues such as H176, R83, and K76 mediate substrates recognition and catalysis through electrostatic interactions and hydrogen bond networks. Functional assays further confirmed the importance of these residues.
In addition, a lipid-like density was observed in the region enclosed by transmembrane helices TM1, TM3, and TM8. Structural analysis and molecular dynamics simulations identified this lipid as phosphatidylserine (PS). PS enhances substrate binding and catalytic efficiency by stabilizing the partially open conformation of G6PC1.
Using techniques such as fluorescence-detection size-exclusion chromatography (F-SEC), the researchers also systematically investigated the molecular mechanisms of pathogenic mutations associated with glycogen storage disease type 1a (GSD-1a). Based on their locations in the protein structure, these mutations were classified into three categories: Class I mutations (e.g., K76N, R83C, H119L) directly disrupt substrate binding or catalysis; Class II mutations are located in the extracellular domain (ECD), potentially impairing the glycosylation and protein folding process; and Class III mutations occur in the transmembrane domain (TMD), destroy the stabilization of hG6PC1.
This study provides detailed molecular insights into how G6PC1 recognizes different substrates, undergoes conformational changes, and carries out catalysis. It also systematically elucidates how disease-associated mutations impair enzyme function. Furthermore, it is the first to reveal a regulatory role for PS in G6PC1 activity.
These findings lay a structural foundation for rational drug design targeting GSD-1a and establish a drug development framework based on the PS-binding site, opening new avenues for treating glucose metabolism disorders.
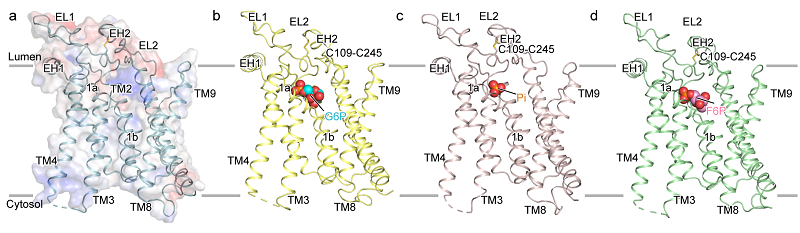
Figure 1. Structures of G6PC1 in the apo state and in complex with different ligands
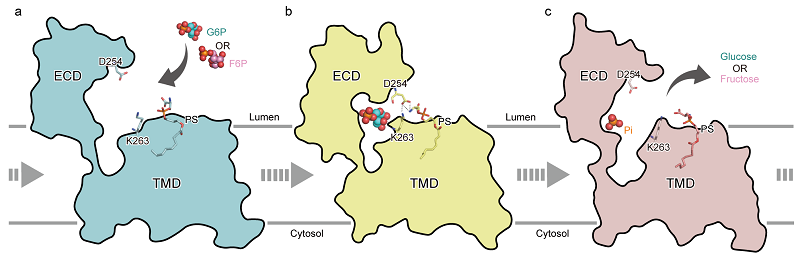
Figure 2. Proposed model of the catalytic hydrolysis mechanism of G6PC1
(Image by ZHAO Yan's group)
Article link: https://www.nature.com/articles/s41421-025-00814-z
Contact: ZHAO Yan
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: zhaoy@ibp.ac.cn
(Reported by Prof. ZHAO Yan's group)

