UPR Promotes Exosome Release Under Stress
Exosomes, small extracellular vesicles released when multivesicular bodies (MVBs) fuse with the plasma membrane, play a crucial role in intercellular communication by transferring proteins, lipids, and nucleic acids between cells. Their secretion is tightly regulated and often influenced by cellular stress conditions. A key question in the field has been how cells under endoplasmic reticulum (ER) stress engage this non-conventional secretory pathway.
On July 21, 2025, a research team led by Prof. WANG Likun from the Institute of Biophysics of the Chinese Academy of Sciences, published a study in the Journal of Biological Chemistry revealing that ER stress-induced activation of the unfolded protein response (UPR) - specifically through the PERK and IRE1α pathways - modulates lysosomal function to promote exosome release in mouse melanoma cells.
The study demonstrates that activation of PERK and IRE1α under ER stress reduces lysosomal acidity and suppresses the fusion of MVBs with lysosomes. This diversion prevents the degradation of MVBs, allowing them instead to fuse with the plasma membrane and release exosomes.
Using thapsigargin (Tg) to induce ER stress, the researchers found that knocking out IRE1α markedly reduced extracellular vesicle (EV) secretion. In contrast, PERK knockout had little direct effect on EV release. However, PERK deletion led to a notable increase in XBP1s protein, a downstream effector of IRE1α, suggesting that IRE1α may compensate for the loss of PERK during ER stress. These findings indicate that both PERK and IRE1α contribute to exosome secretion in a cooperative manner under stress conditions.
The team further showed that PERK deficiency increased lysosomal acidity and enzymatic activity, promoting MVB-lysosome fusion and accelerating MVB degradation - which in turn suppressed exosome release. To probe whether PERK activation alone could drive exosome secretion, they treated cells with ionomycin, which elevates cytosolic calcium levels and selectively activates PERK. The results revealed that PERK activation, even in the absence of ER stress, was sufficient to enhance exosome production.
Similarly, deletion of IRE1α also led to increased lysosomal acidity and degradation activity, thereby reducing exosome output - although the underlying molecular mechanisms remain to be clarified.
These findings uncover a novel mechanism by which the UPR pathways PERK and IRE1α regulate exosome secretion, showing that ER-derived stress signals can reshape the fate of intracellular vesicles by tipping the balance between degradation and secretion. This work adds a new layer of complexity to our understanding of how cells adapt and communicate during stress.
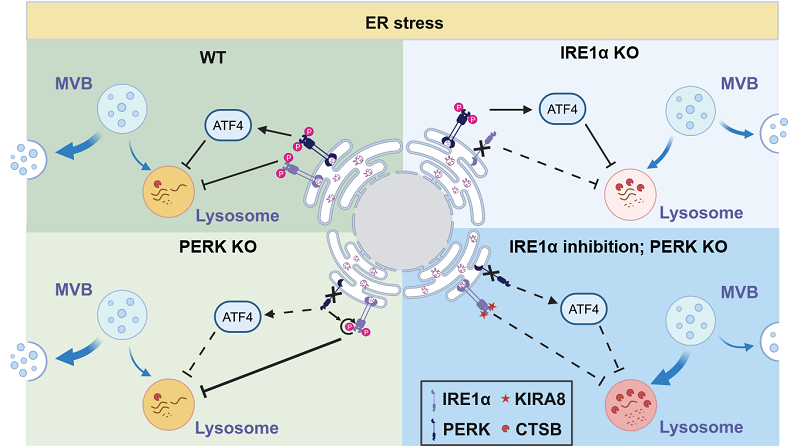
Figure:
Top left: Under ER stress conditions, activation of UPR effectors IRE1α and PERK reduces lysosomal acidity and activity, inhibits MVB-lysosome fusion, and promotes exosome secretion.
Top right: Under ER stress, IRE1α deficiency increases lysosomal acidity and activity, thereby suppressing exosome secretion.
Bottom left: Under ER stress, PERK deletion leads to compensatory activation of IRE1α, which offsets the loss of PERK and maintains exosome secretion.
Bottom right: In cells lacking IRE1α under ER stress, PERK deletion further enhances lysosomal acidity and activity, promotes MVB-lysosome fusion and MVB degradation, ultimately leading to reduced exosome secretion.
(Image by WANG Likun's group)
Article link: https://www.jbc.org/article/S0021-9258(25)02354-3/fulltext
Contact: WANG Likun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: wanglikun@ibp.ac.cn
(Reported by Prof. WANG Likun's group)

