Structural mechanisms underlying the free fatty acid-mediated regulation of DIACYLGLYCEROL O-ACYLTRANSFERASE 1 in Arabidopsis
As plant-based oils serve both food and biodiesel production, the global demand for plant oils is rising rapidly due to continuous population growth and the increasing depletion of fossil fuel resources. Triacylglycerol (TAG) is the main component of plant oils, and its yield and fatty acid composition are crucial for the production of biodiesel and food. Diacylglycerol O-acyltransferase 1 (DGAT1) is a key rate-limiting enzyme in the biosynthesis of TAG, catalyzing the reaction of diacylglycerol (DAG) and acyl-CoA to form TAG and CoASH. DGAT1 is an important target for genetic engineering of plants to increase oil yield and improve oil quality. In addition, DGAT1 plays a crucial role in the plant response to abiotic stress. Although the three-dimensional structure of human DGAT1 have been resolved, the structure of plant DGAT1 and the molecular mechanisms underlying catalysis and activity regulation remain unclear.
On October 13, 2025, the research group lead by Prof. LIU Zhenfeng from the State Key Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences published a study titled "Structural mechanisms underlying the free fatty acid–mediated regulation of DIACYLGLYCEROL O-ACYLTRANSFERASE 1 in Arabidopsis" in The Plant Cell journal. This group resolved the high-resolution cryo-EM structures of wild-type and low-activity mutant H447A of Arabidopsis DGAT1 (AtDGAT1), revealing for the first time the binding sites of two substrates (DAG and oleoyl-CoA), two products (TAG and CoASH), and multiple FFA molecules in DGAT1 (Figure 1).
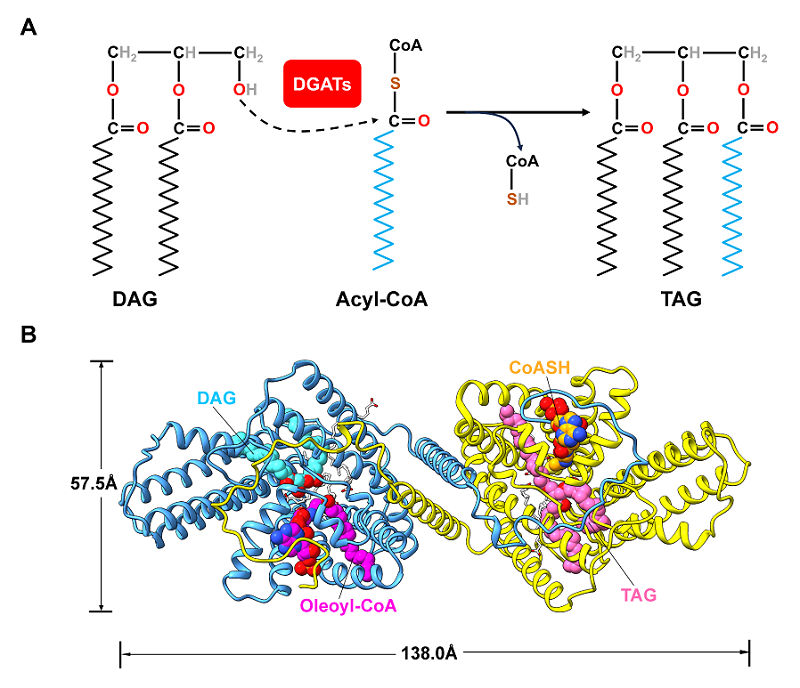
Figure 1. Cryo-EM structures of the AtDGAT1-H447A mutant in complex with OLA, substrates, and products.
(Image by LIU Zhenfeng's group)
This study analyzed the activation effect of different free fatty acids (FFAs) on AtDGAT1 and found that FFA could increase its enzymatic activity by approximately three times. After site-directed mutagenesis of the Cys246 residue interacting with the carboxyl head of FFA (C246A/S/T), the activity of AtDGAT1 was significantly enhanced. The carboxyl head of FFA in the C246A mutant was more deeply located in the active site, enhancing substrate binding. The AtDGAT1-H447A mutant exhibits a pseudo-C2 symmetry, yet the two monomers accommodate distinct ligands at their active sites. Remarkably, one monomer binds the substrates DAG and oleoyl-CoA, whereas the other binds the products TAG and CoASH. In the monomer bound to DAG and oleoyl-CoA, several elongated tubular densities fit well with OLA. Two OLA molecules (OLA-1 and OLA-2) form extensive interactions with oleoyl-CoA, DAG, and nearby residues, while in the monomer bound to TAG and CoASH, a single OLA molecule (OLA-3) interacts with TAG and surrounding residues. Structural analysis showed that the FFA molecule could stabilize the acyl group and thioester bond of the substrate oleoyl-CoA in a position favorable for catalysis. The acyl group of oleoyl-CoA caused conformational changes in TM9, driving the conformational change of Trp366 and stabilizing the glycerol backbone of DAG with the help of water molecules. In the AtDGAT1-H447A complex structure, the N-terminal region of one monomer extends along the cytosolic surface of the adjacent monomer in a domain-swapping mode. Together with molecular dynamics simulations, this suggests that the N terminus allosterically regulates oleoyl-CoA recruitment, binding, and CoASH releasing.
Based on the above results, we proposed a catalytic cycle of acyl transfer mediated by plant DGAT1 (Figure 2): Acyl-CoA is enriched by the N-terminal domain and enters the active site from the cytosolic opening, DAG enters from the lateral opening in the membrane; After Acyl-CoA and DAG are stabilized in the catalytic center with the assistance of FFA, His447 may deprotonate the hydroxyl group of DAG through water molecules and then launch a nucleophilic attack on the thioester bond of acyl-CoA to generate TAG and CoASH; CoASH may be released from the cytosolic side after allosteric regulation from the N-terminal domain of the adjacent monomer, and TAG enters the membrane from the lateral opening; finally, DGAT1 returns to the initial state without substrate binding.
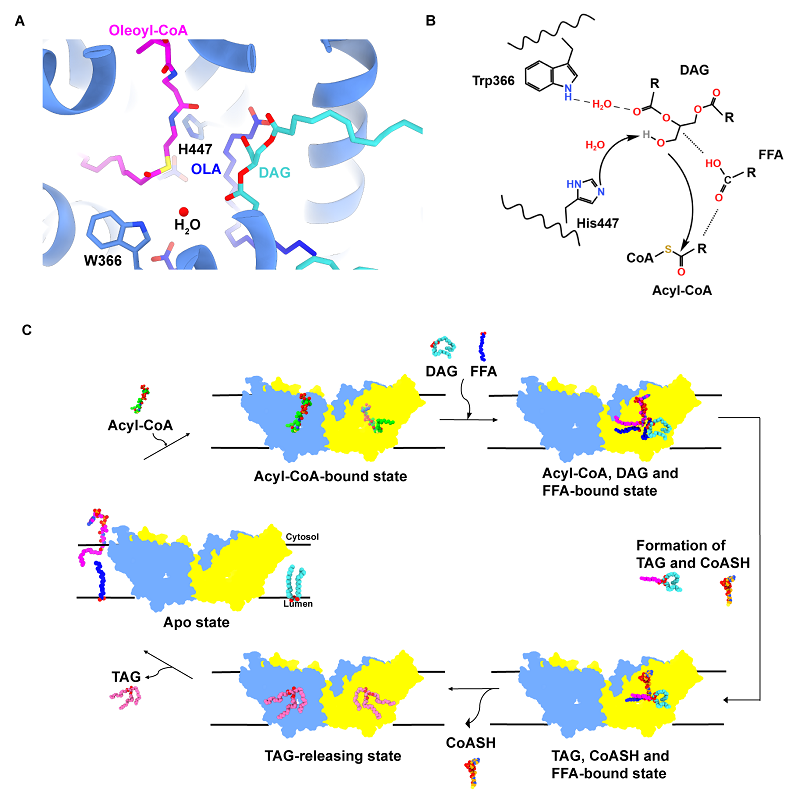
Figure 2: A putative model accounting for the catalytic cycle of DGAT1.
(Image by LIU Zhenfeng's group)
By integrating biochemical data with high-resolution cryo-EM structures, this study elucidates the catalytic mechanism and free fatty acid–mediated regulatory mechanism of plant DGAT1, providing a solid structural foundation for future crop engineering efforts aimed at improving oil yield and lipid composition.
Article link:
Contact: LIU Zhenfeng, LIU Xiuying
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: liuzf@ibp.ac.cn, xiuyingliu@ibp.ac.cn
(Reported by Prof. LIU Zhenfeng's group)

