Researchers Reveal Synergized Antibacterial Mechanisms of Nanozyme with Probiotics by Modulating Tryptophan Pathway
Researchers have revealed a novel mechanism that iron-sulfur nanozyme catalyzes the production of an indole derivative, indole-2-carboxylic acid, in Lactobacillus vaginalis leading to a synergistic antibacterial activity that protects mice and weaned pigs against Salmonella Typhimurium infection.
This study was published in Nature Microbiology on 24 November 2025. The research was co-led by Professor GAO Lizeng from the Institute of Biophysics of the Chinese Academy of Sciences, and Professor DONG Bing from the College of Animal Science and Technology at China Agricultural University.
Gut infections usually result in an altered microbiota with invading pathogenic bacteria and reduced abundances of beneficial commensal bacteria, resulting in gastrointestinal disease. While probiotics offer a potential treatment, their application is hindered by competition with indigenous bacteria, insufficient dosage relative to pathogens, and antibiotic-mediated killing. The laboratory of Professor GAO lizeng had previously showed that iron-sulfur nanomaterials (nFeS) can kill pathogenic bacteria, with three antibacterial modes, namely, catalysis, ferroptosis-like death, and polysulfidation, making it a promising alternative to antibiotics. However, how probiotics interact with nFeS and their impact on pathogens remain unclear.
In this study, the researchers uncovered a protective synergy between nFeS and tryptophan derivatives against gut infections in mice and pigs. The activity was selective that nFeS inhibited intestinal pathogens but spared the commensal Lactobacillus vaginalis (L. vaginalis). Importantly, the presence of L. vaginalis potentiated the ability of nFeS to combat Salmonella enterica serovar Typhimurium in vivo.
Researchers further elucidated the mechanism in vitro. They revealed that the synergistic antibacterial effect between nFeS and I2CA is I3C-dependent and pH-responsive. Briefly, nFeS exhibits oxygenase- and isomerase-like activities, which catalyse the conversion of indole-3-carboxaldehyde (I3C), which is a tryptophan derivative from L. vaginalis, into indole-2-carboxylic acid (I2CA). At the colonic pH, I2CA acts synergistically with nFeS against pathogenic bacteria. Furthermore, inside the probiotics, I2CA can chelate with ferrous ions released from nFeS, thereby rendering nFeS biocompatible. Furthermore, researchers discovered that the I3C or its precursor-generating pathway was present in the probiotics but not in the pathogenic bacteria.
This study illustrates that nanozymes may enable the modification of established metabolic pathways in bacteria and hosts, facilitating both the discovery of functionally distinct metabolites and direct therapeutic intervention.
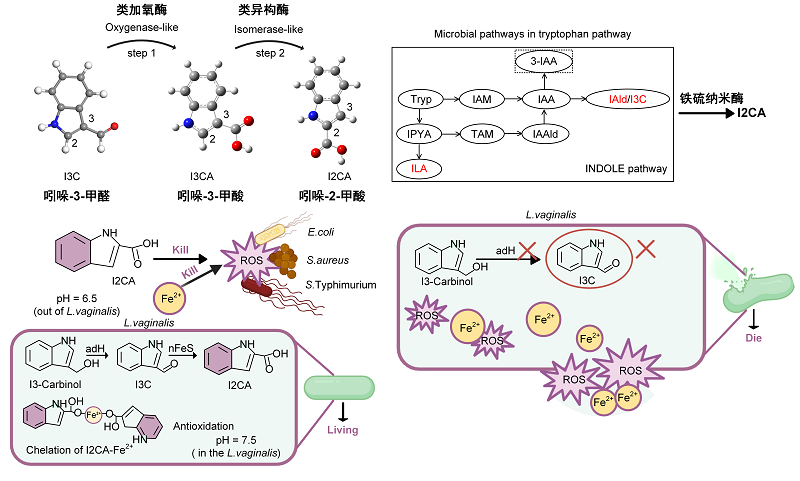
Figure: The schematic that nFeS nanozymes with oxygenase-like and isomerase-like activities modulate microbial tryptophan pathway to generate new indole derivative (I2CA), which enables nFeS nanozymes synergizing with probiotics to kill pathogenic bacteria and improve gut health.
(Image by GAO Lizeng's group)
Article link: https://www.nature.com/articles/s41564-025-02176-4
Contact: GAO Lizeng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: gaolizeng@ibp.ac.cn
(Reported by Prof. GAO Lizeng's group)

