Cells are constantly exposed to stresses from cellular metabolites and environmental genotoxins. DNA damage caused by these genotoxins can be fixed efficiently by DNA repair mechanisms in cooperation with cell cycle checkpoint control; however, unrepaired DNA lesions can lead to cell death, gene mutation and cancer.
Professor Haiying Hang’s group is interested in the cellular roles of a particular group of cell cycle checkpoint genes that is conserved from yeast to humans, namely Hus1, Rad1 and Rad9. Their protein products form a heterotrimeric ring-like complex, called 9-1-1. It is believed that this complex is important for the function of these three proteins in DNA repair as well as in the activation of cell cycle checkpoints. Mutations in these genes lead to malfunctions in cell cycle control and repair of damaged DNA. In a recent paper published in Molecular Cancer1, Prof Hang’s group reports findings from an investigation on the role of Rad1. The Rad1 protein exists in cells as a monomer as well as a component of the 9-1-1 protein complex. While it has been known that Rad1 plays crucial roles in DNA repair and cell cycle checkpoint control, this is the first time that its contribution to carcinogenesis has been investigated.
While constructing mice with a Mrad1 deletion, Prof Hang’s group discovered that Mrad1 null mutants are embryonic lethal. Mrad1+/- mice demonstrated no overt abnormalities up to one and half years of age, but treatment with a combination of DMBA and TPA to induce tumors on mouse skin revealed that Mrad1 is important for preventing tumor development. Tumors were larger, more numerous, and appeared earlier on the skin of Mrad1+/- mice compared to Mrad1+/+ animals. Keratinocytes isolated from Mrad1+/- mice had significantly more spontaneous DNA double strand breaks, proliferated more slowly and had slightly enhanced levels of spontaneous apoptosis than Mrad1+/+ control cells.
Han et al. conclude that Mrad1 is an important genome caretaker or tumor suppressor for skin cancer and probably exerts its role through maintaining genomic integrity. It is likely an essential component for normal embryonic development. The effects of heterozygous deletion of Mrad1 on the proliferation and apoptosis of keratinocytes were different from those they found in a previous study on Mrad9 heterozygous deletion, suggesting that Mrad1 functions independently of Mrad9 in mouse cells in addition to its role in the Mrad9-Mrad1-Mhus1 complex.
1. Lu Han, Zhishang Hu, Yuheng Liu, Xiangyuan Wang, Kevin M Hopkins, Howard B Lieberman and Haiying Hang (2010): Mouse Rad1 deletion enhances susceptibility for skin tumor development. Molecular Cancer 2010, 9:67
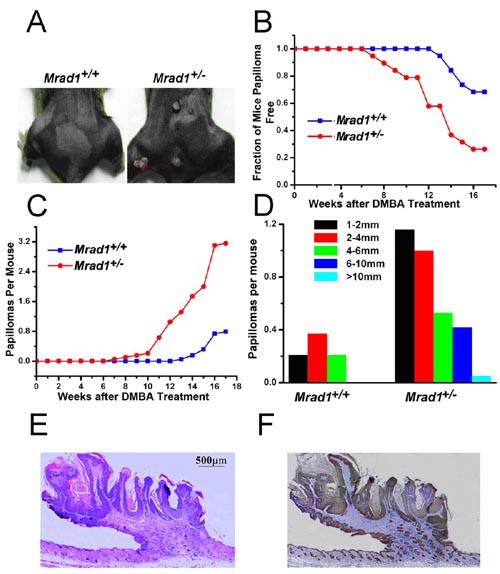
Skin tumor induction by DMBA-TPA treatment. A, Papillomas induced by DMBA-TPA treatment in Mrad1+/+ mouse skin (left) and treated Mrad1+/- mouse skin (right). B, Incidence of papilloma-free mice after DMBA-TPA treatment. Kaplan-Meier plot of tumor-free state as a function of time after DMBA painting followed by TPA treatment (blue, Mrad1+/+; red, Mrad1+/-). Mrad1+/+ and Mrad1+/- mice (n = 38) were initially treated once with DMBA at week 1 on the skin topically starting at ages 7 to 8 weeks, and TPA twice weekly for 17 weeks. There was a significant difference in papilloma formation between Mrad1+/+ and Mrad1+/- mice (P = 0.003). C, Average numbers of papillomas on each mouse (blue, Mrad1+/+; red, Mrad1+/-). Only papillomas larger than 1 mm diameter were counted. There was a significant difference in the number of papillomas per mouse between two of the genotypes at the 17-week end point (P = 0.010). D, Size distribution of papillomas. The length of a papilloma was used to represent its size. E, H & E staining for papillomas. A typical papilloma is shown, with connective tissues extending into the tumor. F, Keratin 14 staining for keratinocytes. The same tumor sample in E was also stained for Keratin 14, and it was thus shown to be derived from keratinocytes.
An interview with Professor Haiying Hang

1. What is the research focus of your laboratory?Tumor development mechanisms derived from genetic defects in genes involved in cell cycle control and/or DNA damage repair.
2. What are the main breakthroughs/achievements your research group has made in the last three years? We found that Rad9 and Rad1 prevented tumor development through stabilizing the genome, that Rad9 plays a direct role in DNA mismatch damage repair through physical interaction with the key mismatch repair protein MLH1, and that Rad1 is able to function not only as part of the 9-1-1 complex (Rad9-Rad1-Hus1), but also independently of Rad9.
3. Where does this paper fit into the story of the work your lab is doing? This paper suggests that Rad1 has independent function(s) beyond its roles through the 9-1-1 complex. Many researchers in the DNA repair and cell cycle control field believe that Rad9, Rad1 and Hus1 play roles only through the 9-1-1 protein complex, but this belief is not backed up by unequivocal experimental evidence.
4. What are the major findings? Although deletion of Rad1 enhances skin tumor development in mice to a similar extent as Rad9 deletions, the molecular mechanism of skin tumor development in Rad1-deleted mice is different. Rad9 deletion activates p53 activities: increased p21 expression, dramatically slows down cell proliferation and elevates apoptosis, while deletion of Rad1 does not. Deletion of either Rad9 or Rad1 increases levels of DNA damage.
5. Can you summarise the significance of your paper in layman’s terms? Rad9, Rad1 and Hus1 are evolutionarily conserved from yeast to humans. They are found to play roles in cell cycle checkpoint control and resistance to DNA damage. Together with Professor Tao Jiang’s group and the Pearl and Cho groups, we recently solved the structure of the 9-1-1 protein complex. Various other lines of biochemical evidence, including results from my research group, support that Rad9, Rad1 and Hus1 forms a 9-1-1 heterotrimer. Most people in the field therefore believe that these three proteins function only through the 9-1-1 complex. In this paper, we’ve shown for the first time that Rad1 has functions that are independent of Rad9, thus suggesting that the 9-1-1 form is not the only form in which Rad1 works. The 9-1-1 complex probably works in some areas and Rad1 probably functions independently in some other cellular activities since Rad9, Rad1 and Hus1 play roles in multiple cellular activities.
6. What’s the next step in this work and do you have a strategy for doing that? We will generate Rad1+/-, Rad9+/-, Rad1+/- plus Rad9+/-, and Rad1+/+ plus Rad9+/+ mice with the same genetic background, and compare their skin tumor development, skin cell proliferation, p53 levels, p53 phosphorylation status, p21 levels and apoptosis levels. We hope to reveal the molecular roles of Rad1 and Rad9 that underly mouse skin tumor development through these experiments.
7. Where do you see your research leading in the future? Rad9, Rad1 and Hus1 are known to play roles in cell cycle checkpoint and DNA repair. They play these roles through both known and as yet unknown pathways. We want to investigate how many pathways are involved and why there is a need for multiple pathways.
8. What are the implications of your research for society? Defects in cell cycle control and DNA repair are two major mechanisms in tumor development. Dissecting how exactly defects arise and finding ways to intervene will help in the development of more efficient tumor therapies.
9. What else is your lab working on now? We are developing technologies for functionalizing and/or optimizing mature proteins and newly designed proteins. We hope to generate antibodies, peptides and enzymes that can be applied in therapy, diagnostics and other areas. Specifically, we are developing in vitro protein evolution processes that mimic biological evolution processes, but offer much faster rates of gene mutation and functional selection.
10. How did you get into this area of research? Major industries have been built up from the knowledge obtained through the study of physics and chemistry. However, discoveries in biology have not yet led to the development of a major industry. Biological systems are much more complicated in form and at the molecular level. Bioparts such as proteins cannot be simply designed and manufactured like mechanical, electronic and optical parts. In nature, new proteins (genes) are functionalized over a very long time through the evolutionary process (mutation plus selection). These processes can be mimicked in a laboratory through so-called ‘in vitro evolution’, allowing designed proteins to be functionalized through a much faster process of mutation and selection.
11. Which of your professional achievements brings you the most satisfaction? The discovery that Rad9 is a key protein in DNA mismatch damage repair.
12. What are the big issues/outstanding problems that have to be addressed in your field? How do the many proteins/genes involved in cell cycle control and DNA repair coordinate their roles to maintain genomic integrity?