Structural basis for the cyclic electron transport in cyanobacteria
Photosynthetic electron transport can be mainly classified into two types, the linear electron transport/flow (LET/LEF) and the cyclic electron transport/flow (CET/CEF). The LEF process produces both ATP and NADPH, which are required for the CO2 assimilation during the Calvin-Bensen-Bassham cycle and other cellular metabolisms. However, LEF alone generates insufficient ATP to balance the ATP/NADPH consumption for CO2 assimilation, whereas CEF has important physiological significance by increasing the produced ATP/NADPH ratio. During the CEF process, the electrons are recycled between PSI and cytochrome b6f through plastoquinone (PQ) pool, simply creating the transmembrane proton gradient, which allows only ATP production without generating NADPH.
In cyanobacteria, one of the type-I NAD(P)H dehydrogenase-like (NDH-1) complexes, namely NDH-1L, is crucial for CEF and respiration. NDH-1L couples the electron transport from ferredoxin (Fd) to plastoquinone (PQ) and proton pumping from cytoplasm to the thylakoid lumen that drives the ATP production. Cyanobacterial NDH-1L contains 19 subunits, including eight oxygenic photosynthesis-specific subunits, and form a similar L-shaped architecture as respiratory complex I. In 2019, two independent research groups reported the structures of cyanobacterial NDH-1L complex, revealing the overall architecture and locations of 18 subunits, but lacking one subunit NdhV. Moreover, the exact binding site of Fd and PQ in cyanobacterial NDH-1L, as well as their interacting pattern with NDH-1L complex remain to be elucidated. The structural analysis of cyanobacterial NDH-1L bound with Fd/PQ is crucial for understanding the molecular mechanism of CEF process.
CHANG Wenrui - LI Mei's group and ZHANG Xinzheng's group from the Institute of Biophysics (IBP), Chinese Academy of Sciences (CAS), collaborated with MI Hualing's group from Institute of Plant Physiology and Ecology, CAS, solved two structures of NDH-1L from T. elongatus BP-1 by single particle cryo-electron microscopy (cryo-EM) method, one bound with an endogenous PQ (NDH-PQ), and the other bound with Fd (NDH-Fd), at resolutions of 3 angstrom and 3.2 angstrom, respectively. In the NDH-PQ structure, one PQ molecule was discovered in the interface of the membrane and hydrophilic arm of NDH-1L. The NDH-Fd structure contains all 19 subunits and shows the detailed interacting pattern between Fd and NDH-1L. This work provides the complete structural model of NDH-1L containing NdhV, unravels the binding pocket of Fd and PQ, and reveals the electron transport path from Fd to PQ. These results provide important molecular basis for understanding the coupling between electron transport and proton pumping of NDH-1L.
The research work, entitled "Structural basis for electron transport mechanism of complex I-like photosynthetic NAD(P)H dehydrogenase" (DOI: 10.1038/s41467-020-14456-0) was published in Nature Communications on Jan. 30th, 2020. This research was supported by the National Key R&D Program of China, the Strategic Priority Research Program of CAS, the Key Research Program of Frontier Sciences of CAS, National Natural Science Foundation of China and the Youth Innovation Promotion Association of the CAS. The cryo-EM data was collected in Center for Biological Imaging, IBP, CAS. The sample analysis work was assisted by the staffs in Chinese Academy of Sciences Protein Science Core Facility Center.
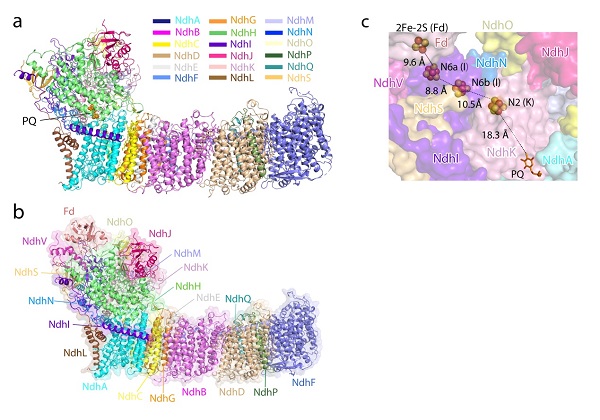
Figure. Overall structures of NDH-PQ (a) and NDH-Fd (b), and the cofactors involved in the electron transport chain (c).
(The image by Dr. LI Mei's lab)
The web link for this paper is https://www.nature.com/articles/s41467-020-14456-0
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-6488511
Web Site: http://english.ibp.cas.cn/ibp_pi/L/201902/t20190228_205789.html
(Reported by Dr. LI Mei's group)

