Structural basis for energy transfer and electron transport of cyanobacterial photosystem I under iron-deficiency conditions
Cyanobacteria flourish in abundance throughout most marine and fresh waters. They contribute significantly to the primary production in the aqueous environment through photosynthesis, and are studied extensively as an ideal model organism in research of photosynthesis.
Iron-deficiency stress occurs frequently in natural aquatic environments, and cyanobacteria adapt to the iron-stress through reducing the amount of iron-enriched proteins, including photosystem I (PSI) and ferredoxin (Fd). To compensate for the reduction in PSI and Fd content, cyanobacteria upregulate the expression of iron stress-induced protein A(IsiA) and flavodoxin (Fld).
Eighteen IsiA proteins function as the peripheral antennae encircling the PSI core, whereas Fld replaces Fd as the electron receptor at the cytoplasmic side of PSI, forming PSI–IsiA and PSI–IsiA–Fld supercomplexes, therefore enabling normal growth as well as ensuring photosynthetic efficiency of cyanobacteria.
In a new study published on Feb. 10th in Nature Plants, a research team led by Professors LI Mei and ZHANG Xinzheng of the Institute of Biophysics, Chinese Academy of Sciences, has reported single particle cryo-electron microscopy (Cryo-EM) structures of cyanobacterial PSI–IsiA and PSI–IsiA–Fld supercomplexes from Synechococcus sp. PCC 7942 at resolutions of 2.9 angstrom and 3.3 angstrom, respectively. These structures provide detailed information for deeper understanding the energy transfer and electron transport of cyanobacterial photosystem I under iron-deficiency conditions.
The high-resolution structures reveal notable structural variations and novel pigment-binding sites of PSI core subunits compared with those from other cyanobacterial species.The established potential multiple excitation energy transfer pathways in the PSI–IsiA supercomplex explain the efficient energy absorption and transferring of IsiA antennae to the PSI core.
The PSI–IsiA–Fld structure unravels the binding site of Fld in the PSI core, and shows the detailed interaction pattern and the electron transport path between PSI and Fld. This research reveals new features and a sophisticated pigment network of PSI–IsiA–Fld supercomplex, and provides detailed structural data for understanding the mechanisms of light harvesting, energy transfer and electron transport of cyanobacterial PSI under iron-stress conditions.
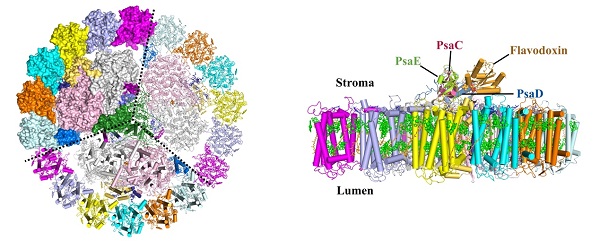
Figure legend:
Left: Cytoplasmic view of the PSI-IsiA supercomplex. The cytoplasmic subunits of PSI core are omitted for clarity. Dashed lines separate three monomeric complexes of the homotrimer. Right: Side view of the PSI-IsiA-Fld supercomplex. Only one monomeric complex is shown for clarity. The cytoplasmic subunits of PSI core (PsaC, PsaD and PsaE) and Fld are labelled.
(The image by Dr. LI Mei's lab)
The web link for this paper is https://www.nature.com/articles/s41477-020-0593-7
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-6488511
Web Site: http://english.ibp.cas.cn/ibp_pi/L/201902/t20190228_205789.html
(Reported by Dr. LI Mei's group)

