Catalytic Mechanisms and Redox Regulation of the Calvin-Benson-Bassham Cycle of Photosynthesis
Photosynthesis can be divided into light reactions and the Calvin-Benson-Bassham (CBB) cycle (light-independent reactions). ATP and NADPH are produced in the light reaction process before being utilized by the enzymes of the CBB cycle responsible for CO2 assimilation. Though the CBB cycle is light-independent, it is regulated by the light/dark transitions. After a light signal passes through various proteins in the light reaction process, a redox signal is generated to regulate the CBB cycle and a number of downstream reactions through chloroplast thioredoxins (TRXs).
Phosphoribulokinase (PRK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are essential enzymes of the CBB cycle as they deplete ATP and NADPH generated in the light reactions, respectively. PRK and GAPDH are both redox-regulated. Reduced PRK or GAPDH represents its active state, while the oxidized form is inactive. In addition, the small chloroplast protein CP12 is also redox-regulated by TRXs, and when oxidized, it can be associated with PRK and GAPDH to form the GAPDH/CP12/PRK complex. After formation of the GAPDH/CP12/PRK complex, both enzymes are further inhabited.
On May 8, 2020, THE PLANT CELL published a report by the Li Mei/Chang Wenrui Project Team at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences (CAS) titled Photosynthetic Phosphoribulokinase Structures: Enzymatic Mechanisms and the Redox Regulation of the Calvin-Benson-Bassham Cycle. The study reveals the PRK catalytic reaction mechanism in the CBB cycle of photosynthesis (light-independent reactions) and provides a structural basis for understanding how the CBB cycle is regulated by the light/dark transitions.
The team determined the crystal structures of PRK from Synechococcus elongatus PCC7942 (SePRK) in complex with adenosine diphosphate (ADP) and glucose-6-phosphate (G6P), and from Arabidopsis in both their reduced (AtPRKred) and oxidized (AtPRKox) forms. Furthermore, the team solved the crystal structure of the AtGAPDH/CP12/PRK complex. Based on the structural information, and in view of the results of mutation and affinity assays, the team determined the PRK active sites as well as the key domains and amino acids involved in catalysis reactions, explained the molecular mechanism by which PRK is redox-regulated and detailed protein interactions in the GAPDH/CP12/PRK complex; they also revealed how CP12 responds to redox signals to regulate PRK and GAPDH activity. This structure-function study greatly advances the understanding of the reaction mechanism of PRK and the subtle regulations of redox signaling for the CBB cycle.
Li Mei from the IBP of CAS is the correspondence author of the report. Doctoral candidates Yu Ailing and Xie Yuan are co-first authors. This work was supported by the Ministry of Science and Technology of the People’s Republic of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, and the National Natural Science Foundation of China. Staff at the Shanghai Synchrotron Radiation Facility and Core Facilities for Protein Science at the IBP of CAS supported the project with data collection and sample analysis.
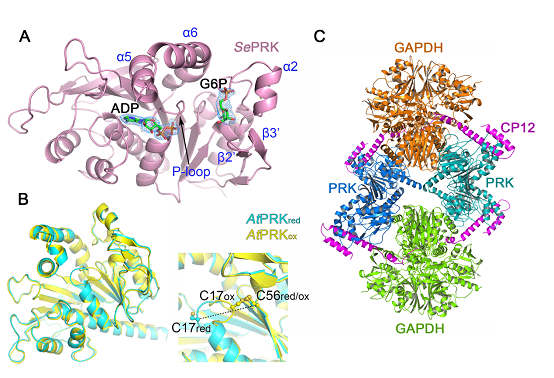
Fig. A SePRK-ADP-G6P structure; Fig. B Structural comparison of AtPRKox and AtPRKred; Fig. C Three-dimensional structure of GAPDH/CP12/PRK complex
(The image by Dr. LI Mei's lab)
The web link for this paper is http://www.plantcell.org/content/32/5/1556
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-6488511
Web Site: http://english.ibp.cas.cn/ibp_pi/L/201902/t20190228_205789.html
(Reported by Dr. LI Mei's group)

